Welcome, this site contains useful information regarding Breast Implant Illness (BII).
The recovery of a particular illness is going to begin with removing any interference with the body. That is when reversal can occur, not from symptomatic treatment which treats the symptoms but not the source.
Thousands of women with breast implants have developed various symptoms in response to the inflammation (foreign body reaction) and toxicity from their breast implants. They began seeking help from their physician and specialists – many adjust their lifestyles to cleaner eating, less work, various health treatments – without resolve until connecting their symptoms to a systemic response to their breast implants regardless of make or model.
It has been established for the past several decades that the first step in recovery is explant surgery to remove the implants and the surrounding scar tissue known as capsules.
Why explant without replacement?
- You have many unexplainable symptoms, such as: fatigue, cognitive dysfunction (brain fog, memory loss), muscle aches, joint pain, hair loss, dryness throughout the body, recurring infections, gastrointestinal and digestive issues, rashes, problems with thyroid and adrenals.
- The occurrence of a new autoimmune disease, with or without family history, or have a preexisting autoimmune disease
- The occurrence of new allergies, food intolerances, or chemical sensitivities
- You’ve gone from doctor to doctor and continue to have unresolved health issues
It can be helpful to write down a chronological list of Symptoms from when they first started. You may notice a pattern of how they began after implantation and have progressively worsened over time.
The first step toward recovery is Explant: choose a plastic surgeon experienced in performing a full capsule removal through en bloc or total capsulectomy, ask for all silicone to be removed in the event there is a rupture or gel bleed, request that the surgeon takes photos of your capsules and implants during the surgery, and have the implants returned to you so you can inspect them. You may also ask the surgeon to take swabs during surgery and send them to Pathology along with the capsules to check for any pathogens (biofilm, bacteria, fungi), inflammatory cells, and/or malignant cells related to BIA-ALCL (most linked to textured breast implants).
Always do your own due diligence in selecting an Explant Surgeon and prepare a list of Questions to Ask Explant Surgeons. The surgeons on this list are added based on word of mouth women’s recommendations and photos of capsules and implants that show en bloc or total capsulectomy. This website receives no money from any surgeons.
You can try to see if Insurance can cover the surgery.
Explant is the key step towards recovery, as it removes the foreign body and toxic interference. After explant you can focus on healing and Detoxification of the body from the accumulated toxins (heavy metals, chemicals, silicone, biotoxins). The body is capable of great regenerative and healing potentials.
Inflammation & Toxicity
Breast implants are a controversial subject that are still advertised as safe by plastic surgeons, doctors, and the medical community when in fact they can cause many health issues. Doctors typically focus discussions on the aesthetics and local complications – without disclosing the persistent foreign body reaction, adjuvant effects of silicone toxicity, heavy metal exposure, how the implants release gel bleed, the development of biofilm around implants (causing persistent low grade chronic bacterial infections, chronic inflammation, capsular contracture, etc), and how the implants are oxidized in the body (causing free radical/oxidative damage). These factors can weaken the immune system and overtime can result in a systemic cascade of health issues; which may include autoantibodies, autoimmune diseases, endocrine dysfunction, thyroid and adrenal problems, gut dysbiosis, increased susceptibility to bacterial and viral infections, and more.
Trust your instincts and listen intuitively to your body, it sends signals through symptoms.
Contact: This website can be reached by comment or email, [email protected].
Disclaimer: Please be advised that the author is not a medical or legal professional and this website is not intended for medical or legal advice, it is for informational purposes only. Please refer to the Legal section for a complete website disclaimer. This is a non-commercial website and does not receive money from any sources.
Hi Everyone. I had implants for many years and learned through this website and FB groups about BII. I explanted in 2017 from a local doctor here in CO. While he did not believe in BII at the time, he performed an excellent surgery, removed the capsule and all was well. My healing journey before and after BII/ Explant has led me to help others. If you need assistance with detoxing or rebuilding a healthy body, I am happy to help. One thing to remember is that removing them is not the same as detoxing. And even if detoxing, you still need to support your body through diet, nutrition, lymphatic drainage and circadian rhythm. I know first hand how difficult healing can be and there are so many of his here to support you.
Hi Alexi,
I had my implants removed 3 months ago. And although I feel so much better I am still feeling off and trying to figure out the next step in my recovery plan. You mention lymphatic drainage and circadian rhythm… do you have any suggestions or resources ?
Attachment BII-POSTER_Completed-Version-on-6th-Feb-2023.pdf
Hi, my name is Marina, and I’m from Japan.
I created this BII awareness poster a few years ago in collaboration with the admin of this website, and I really hope it can help people in English-speaking communities too!
Feel free to download, share, or print it to help spread awareness.
Download here:
https://drive.google.com/drive/folders/1RxZtcw8mChpg3VJaGYQ2D-mVRbv7MYip?usp=drive_link
https://www.dropbox.com/scl/fo/4m4jdrw447ex0j0l15qc8/AFjsq4bUnWFN2DaSY59MPrs?rlkey=vilhi8fpnacjnqkjvmoo7jmil&st=vlpjdubn&dl=0
This poster has been translated into Japanese and has already helped many people in Japan.
I am sharing this with the ME/CFS community, as symptoms of Breast Implant Illness (BII) are often misdiagnosed as Chronic Fatigue Syndrome, and raising awareness could make a real difference.
Feel free to download, share, or print it for awareness events!
Hello. My Name is Keri and I am an admin for a couple breast implant illness groups as well as an active advocate who even spoke at the FDA hearings in 2019. Im trying to find who runs this website and set up the surgeons list. I need to speak with you about one of the surgeons on the list. Multiple women have informed me of one of them leaving residual capsule while claiming to have done enbloc and they had to go back and have residual capsule removed by another surgeon. If you could please email me or you can message me on Facebook. You can find me on my bii support group “breast implant illness support by keri”
Hope to hear from you.
Are there any in Long Island New York? I have no family and don’t know anyone and I’m going through a life-saving surgery with Dr. feingold in great neck on October 14. I really really need support. I’m going to be alone and have been alone. I’ve been in the hospital 20 times in four states and multiple counties 60 doctor visits. I’m basically unable to walk through a supermarket most days I wake up suffocated with my throat so swollen I’ve had them in since 1990. I was just a kid the ones that are the deadliest and we were banned in 1992 and knowing now that they’re all deadly I have been doing my research but I just need a group or someone to go or someone to somewhere to go where I could get some kind of one on one or group personal human contact I am fighting through all this totally alone and will come out of surgery totally alone. I don’t have regular insurance. I’m paying $21,000 out-of-pocket. I only have health first and I’m looking for any kind of support groups so I can make friends and have someone to go through this with. I have volunteered in other areas in life with all the situations after losing my dad to help other people for 20 years my mother volunteered in the church for 34. Why can I get some support? Sincerely Denise Lynn Miller I’m in SAYVILLE Long Island, New York, desperate for human contact and support
Whereabouts of Dr Susan Kolb? I do not believe she is currently practicing but seek her knowledge to help my mystery illness that has been dismissed by multiple drs and specialist. She saved my sister’s life 11 years ago and I’m seeking the same from her knowledge and deep knowing.
Under Resources, we’ve saved some links to her works:
Silicone and Saline Implants Q&A
Doctors, are you listening?
Immune Protocol
Silicone Immune Treatment Protocol
Inositol for Silicone Detoxification Provided by Dr. Douglas Shanklin
Videos: Dr. Susan Kolb discusses silicone breast implants and saline breast implants
Book: The Naked Truth About Breast Implants: From Harm to Healing
Email us and we can provide an email of hers.
Hola,
Escribo desde Trujillo, Perú. Quisiera recomendar a mi doctor por el buen trabajo que hizo. Y para que otras mujeres que se encuentren en la búsqueda como yo lo estuve tengan esta opción, ya que no hay información sobre buenos doctores en Perú.
El Doctor Raul Plascencia Santa Maria, en Trujillo Perú, opera en la clinica Plascencia, excelente profesional! experto en cirugía plástica, y a petición realiza explántación enbloque (siempre que sea posible)
Para las chicas en Perú, lo recomiendo de todo corazón! No se arrepentirán.
Hola! Me puedes ayudar al respecto del asunto de Dr. Raúl? Me gustaría aprender de sus métodos y precios, si me puedas brindar, por dos. Gracias de antemano! Te agradezco un montón cualquier información que compartes conmigo.
Attachment
Somebody have Eurosilicone Ref. 812N
I will explant in June 2024 and I have that Brand and Reference # but I would like to know if they are textured or no. I have the video from the surgery when they implant me, and It looks textured but I dont know
Brenda,
I am new onto this site and I am seeing that you had Euro silicone implants and subsequently BII.
Can you tell me more about this? I unfortunately recently in July of this year had Euro silicone implants placed. Since then, I have not felt myself and am chronically getting worse. I have what was an initially diagnosed as burning mouth syndrome. But have come to the conclusion that it’s BII.
My mouth is stolen fire I have a chronic sore throat swollen lymph nodes intermittent fever and chills tingling in the crown of my head, I am short of breath with minimal activity,numbness in the tips of my fingers and chronic widespread joint pain which is worsening by the day.
If anyone has any input please let me know as this is a self-diagnosis at this point of BII. But I believe that I should move forward with my concerns while my daughter thinks I am overreacting. Someone please reach out
Hello everyone
Thank you for this very informative website.
Please could you upload information on the Motiva implants, they are a cohesive gel. I have had all kinds of symptoms since having my previous implants and now I have new Motiva ones which I’m wondering if it was the right decision as i don’t wish to get sick again 🙁 but the surgeon said my body would be ruined if I explanted and I would be flat chested and scarred so he insisted on replacing the ruptured implant for the new Motiva implants.
Thanks
Carly
Has anyone experienced low platelet count as part of BII? I have had Allergan Textured silicone implants for 11 years. The were voluntarily recalled in 2019 for possible link to BIA-ALCL. My surgeon dismissed the recall as panic and lacking scientific rationale. I have not had any issues other than difficulty doing pec involved exercises. However, two years after implant, a routine blood test showed my platelet count a little lower than normal. Every year since, my platelets have continued to drop. In 2021 my cholesterol was noted to be high and I was started on a low dose statin. The platelets dropped in half. I have been tested for every autoimmune disease under the sun and I have systematically removed all supplements and meds (including the statin) for trials to see if anything is causing the low platelets. No change. My B12 was also very very low but is back up now with first injections and now just a daily oral gel cap. I also take a D3 supplement daily. I am 2 years post menopause and my most recent bone density scan showed a progression from osteopenia to osteoarthritis. Wondering if that could be impacted by my implants as well. I am past 10 years with them and am contemplating removal even though I have not had most of the symptoms associated with BII. My PCP said the removal surgery is really bad if you keep them too long because of calcification. Does anyone have thoughts on that? Many thanks for this open exchange of experiences. And please comment on the platelet count.
I had my allergan textured implants (silicone) since 2010. Had extraction on 8/16/24 – one was ruptured. So glad I decided to have them taken out and not wait for these recalled bags of poison to cause anymore issues!
Did you ever get a reply in regards to platelets?
Note, Dr. John Pierce, who is on the list, no longer does breast augmentation. Practice is approx 95% explant. Has performed thousands, exclusively for the BII community over the past 4-5 years. Does total precise capsulectomy every time, enbloc if possible.
Check out Instagram @plasticsurgeonscottsdale for explant videos, pictures of capsules and before and after pictures.
Check out the explant FAQ on the website under Office tab, patient forms http://www.desertplasticsurgery.com
Thank you!
Christina Pierce
Desert Plastic Surgery
Scottsdale, AZ
Thank you Christina for this update and thank you Dr. Pierce for no longer doing breast augmentations. His information has been updated on the list.
To Whom It May Concern:
I am requesting permission to use the following material from your website:
“…the implants are made out of a concoction of neurotoxic and carcinogenic chemicals and heavy metals that slowly breakdown and accumulate in the body, causing an overload of toxicity. All implant shells are made out of silicone and are semi-permeable. Upon implantation they release heavy metals, silicone, and chemicals that can migrate and be stored throughout the body. Silicone is an adjuvant and an endocrine disruptor. It is an internal irritant that has the ability to modulate immune, endocrinological, and neurotransmitter functions. The silicone that leaks out of breast implants is in the low molecular form which is very toxic to the body. Its widespread effects induce silicone toxicity and can impair many functions. The exposure to toxic chemicals causes immune disruption (autoimmunity, allergies, recurring infections), accelerated aging, neurological symptoms, and more.
Overall, the chronic stress of the foreign body reaction, silicone toxicity, heavy metal exposure, and gel bleed result in a weakened immune system, buildup of implant toxins, free radicals inducing oxidative stress, and vulnerability to opportunistic pathogens (bacteria, fungi, parasites). Immune dysfunction allows opportunistic pathogens to grow out of control (ex. candida) and for dormant viruses to reactivate (Epstein-Barr virus, cytomegalovirus, etc). A cascade of systemic dysregulation eventually develops. In addition, saline implants can have faulty valves and cultivate mold and microorganisms. Detoxification can be increasingly impaired as the liver, kidneys, and other excretory organs struggle to remove toxins. The body goes into a systemic state of chronic inflammation. All of this adds up to a slowly developing chronic debilitating illness affecting many organ systems of the body.”
I would like to include the above material as part of a book I am preparing and hoping to publish during the summer of 2023.
I request your permission to use this material in my work, in all languages and for all editions and formats, including digital/electronic. I assure you that proper credit will be given to breastimplants.org. If you agree, please let me know in writing, via email.
I would be very grateful for your permission.
Hi Adriana, yes that is fine. As a note, this website is breastimplantillness.com not breastimplants.org.
Thank you so much!
Attachment
I just had my explant surgery on June 9th. Saline over filled 960 cc. 36DDD bra!! For the past years I have had chronic fatigue, increase yeast in my body, horrible acne and many other things
View image
Sending you warm wishes and a big virtual hug!! It must feel good to have those weights off your chest and to be able to take a deep breath. Take the first month easy, listen to your body, and remember healing takes time. Wishing you a speedy recovery. <3
Hi, my name is Laura Bowden. I am a BII survivor, also a BII Advocate for over 30 yrs. I would love to mention another amazing Surgeon named Dr. Aditya Sood at Pryorhealth in Rockford Illinois. He nog only believes in BII, he performs and understands the importance of removing the scar capsules with every explant. He truly is a hidden gem and I would to see him added to this list of wonderful Surgeons if possible. Thank you!
Thank you, Laura. Do you know Dr. Sood’s and Dr. Pryor’s explant prices?
For our reader’s, Laura is an amazing longtime BII advocate and pioneer. Laura works for Dr. Pryor as his BII patient advocate, here is more about her from Pryor Health:
Laura is a Breast Implant Illness Survivor (since 1992) and a bit of a “Pioneer” for BII. Her goal is to educate and prevent future generations that are suffering from this injustice and help guide others who have been affected. She has appeared on the Oprah Winfrey Show, Jenny Jones, and local Chicago news to advocate for women’s health and wellness. She had the honor to speak in front of the FDA, regarding the dangers of breast implants, with 80 incredible BII Advocates in March of 2019. When Laura is not working, she enjoys spending time with her family, grandbabies, and friends.
Can implants cause cytolytic vaginosis? It’s an overgrowth in lactobacillus. I have autoimmune issues. I will be doing an explant this year. Does anyone have any information on fat transfer and if it is safe for women that have autoimmune disease?
Attachment BII-POSTER_Completed-Version-on-6th-Feb-2023-1.pdf
Hi! I have made BII Poster. Please use this poster to raise awareness about BII. Please edit this poster to translate into another languages.
Hello. Thank you for the poster. Sorry cannot see it.
I would love to see this poster as well! Can you post so we can see it? Thanks so much 🙂
Poster
I had my implants for 7.5 years. Strange and non-specific symptoms started about 2-3 years after. I am a rather healthy 30 some year old and I first experienced shingles. I assumed it was from stress and not sleeping well. Then at work, all of the sudden I had shaking, dizziness, tachycardia that came and went. It got so bad I had to leave work and I went to the ER. I received fluids, but they did blood work and EKG and didn’t find anything. Also, I’ve always had palpitations, but they were getting worse. I told my doctor what happened and he ordered me a Holter Monitor to wear for 3 days to make sure there wasn’t anything cardiac going on. The results were negative for any issues. I had another “episode” a few years later at work, again, and I thought it was my blood sugar, but it wasn’t. I was shaky, tachycardia, dizzy, etc… I got past it but a few days later it happened again and I ended up in the ER. Nothing was found. I went to my primary care and she ordered a lot of blood work. Negative for everything, covid, flu, mono, everything was normal, including iron study, except my vitamin D was a little low. I started taking vitamin D. But I was still tired and I always felt this “on edge” feeling and that something didn’t seem right. I looked in to POTS symptoms and I self-diagnosed myself and started increasing fluid and salt intake. I also developed this place on my forehead that was red and irritated and was extremely sensitive to light. I also developed random rosacea. The red patch went away and so did one patch of rosacea, but there was still a big patch on my face. I randomly got itchy spots on my skin that would go away but come back at random times. My friends suggested seeing a functional medical doctor. They have done most every test under the sun, hormone, saliva, urine, mycotoxin (which was elevated), blood tests, levels of every kind and mostly were normal except my ANA (anti-nuclear antibody) and SLC-70 (scleroderma) titer. I had an ANA of 1:60 and a positive SLC-70. I don’t have any specific symptoms of scleroderma. I was very upset and distraught. I couldn’t understand why I had positive titers and not have specific symptoms. I was just waiting and waiting for it all to develop at once. I’ve always been a clean, healthy eater and exercise regularly. I am a huge health advocate. When I went to the functional doctor, I eliminated more foods, sugar, went paleo, took a ton of supplements. I felt better, but not all the way better. I was so fatigued and felt always in a fog. I see now how much of a brain fog I was in constantly and how tired I was now that I have my implants out. I started finding information on BII. I started putting pieces to this mysterious puzzle together. I first went to a consultation with my plastic surgeon at the beginning of the year to get them out and he told me BII was not a thing. Finally, months later after having another “episode” in church, I made an appointment to get them out. I am a month out and I feel amazing. I know I have some healing to do, but I am hoping and praying that the autoimmune markers are down and hopefully one day gone. I have so much more energy. I can think clearly. My skin started producing oil again. I feel like my old self. I wish I NEVER would have had these toxic bags put in. I didn’t even care that I was flatter than a board. I just had a friend do it and she said it was super simple and affordable so I went to a consultation. Please, if you are considering breast implants, PLEASE DON’T. Even if it is just for fun or you aren’t happy with the way you look…. Love the body God gave you.
Correction to my above comment: I had an ANA of 1:160, not 1:60.
Although one month after explant, my ANA is 1:320 and SLC-70 still positive. My brain fog is gone and my energy is still improved. Overall, I still feel much better.
Also, has anyone had a similar experience with positive ANA and SLC-70? If so, has anyone had the experience of the markers decreasing or eventually negative?
Do you mind letting me know if you were vaccinated for Covid and how your symptoms correlated? I didn’t have any symptoms for four years with my implants until my Covid vaccine for work and then everything happened almost identical to what you’re saying and I have POTS now secondary to the vaccine.
Unfortunately I did get the COVID vaccine since I worked at a hospital. I unfortunately got the first two vaccines and then never again. Some of my symptoms were before COVID, but I agree it seems like things started to accelerate after the vaccine.
I also am grateful to say several years later I had my ANA tested again, and it was absolutely negative! Someone I worked with had a friend who had a positive ANA with implants and then had them taken out and her ANA was negative later as well.
Found in the women’s bathroom at the Museum of Science and Industry in Chicago. Thank you for sharing.
Hi sister
my name is wal
I’m DM from @perigos.do.silicone (Brazilian explant ig)
I have an idea to open a WhatsApp with the main activists in the world so that we can share information and help each other.
Would you be interested in participating?
If so, send me a message on Instagram
Thank you and have a nice day!
Wal
How can you find out what kind of implants you have? I had mine in 1982. There are no local available records that go back that far.
Implants from 1982 should have already been removed. I had my first set put in 1991 and they said implants are technically only supposed to last 10 years but you may get 15 out of them. I would say yours are way beyond.
Thank you for this site. I am a breast cancer survivor and did a single mastectomy with saline implant 7 years ago. I am ready to explant to flat and had my first consultation a couple days ago. The plastic surgeon is on the list of recommended doctors and said he will try his best to do an en bloc/total capsulectomy but can’t guarantee he will be able to get the entire capsule because it is difficult when removing it from under the muscle where it attaches to the rib cage without damaging the area. He said he can for sure get 93-94% of the capsule and will cauterize the remaining area if need be. He also washed out the area with antibiotics and some type of acid solution (not sure of the name) is put on the area for 2 minutes to remove biofilm. This seems reasonable but I really hope the entire capsule can be removed since my BII has been debilitating. The estimate says capsulectomy with explanation and excision of encapsulated mass in left breast. He has great reviews and has done 225 explants. How do I request the wording says Total Capsulectomy when he said he can’t promise this? It also sounds like I will be his first breast cancer survivor explanting and with it only being one breast, it should only take an hour. This seems fast. Are these things I should be concerned about? Thanks so much for all of the helpful information on here and any advise others can offer!
Hi Cindy,
Experienced surgeons can do total capsulectomy and aim for en bloc but en bloc is not always possible. It generally takes two hours to do proper explant on both breasts; since you’re doing explant on only one breast one hour is reasonable. Can you comment or email us who the surgeon is?
Thank you very much for this information. The surgeon is Dr. Spies in Arizona and is on recommended lists that I’ve seen. He spent a lot of time with me during the consultation and said 9 out of 10 of his patients feel better after doing the explant.
I had Natrelle Inspira gummy bear silicone implants put in 9/21/2016 and I started getting symptoms 2 years later. I was diagnosed with hypothyroidism in 2018, Hashimotos thyroiditis (autoimmune disorder) 2020 and I was in and out of the ER constantly with vertigo symptoms, infections, unexplainable rashes, hormone issues, dry eyes, hair loss but tests would come back normal. I was always tired, it was a fatigue that I was going to pass out from exhaustion when I didn’t even do much. I had muscle weakness so after being an athlete and coach my entire life, I couldn’t push my self to workout more than 2 minutes. I had my explant surgery March 24, 2022 and immediately after waking up from surgery I felt energized, migraines were gone, and most symptoms were cured. I am detoxing now and my arm pits smell like copper metal. I know that my thyroid and autoimmune will take time and might not be healed, but I can say just having energy to do things is a HUGE thing. I feel like I’m alive again. I hope and pray that women will figure this out IF the implants are the cause of them being sick. I had 38 symptoms from bowel issues, menstrual issues, NO libido at all, dizziness, cold intolerance…. and most of those are completely gone.
Hi. I was explanted two days ago same symptoms as yours. But don’t feel any difference yet
Hi ladies, I had mentor saline DV IMPLANTS that were on a field safety recall list issued last October, I’m in Idaho, I had the implants for 20 years, recently explanted. The last ten years of my life have been hell, seven surgeries and sick all the time. I’m looking for a national attorney that will take my case, I know there are class action suits, but I’ve not yet been able to find anyone to help. Will someone please help me? I haven’t worked in two years, I am a year and a half into disability (now at hearing stage) I feel like giving up, but believe I’ve truly been wronged.
Hello,
I am a breast cancer patient doing my research.
I found a helpful resource:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm.
I used Simple Search for an implant name and symptoms, for example ‘Mentor saline pain’
Whereas there are many reports of breast implant illness symptoms, Ideal implants are only associated with deflation or capsular contracture.
Does it mean that Ideal implants are better than other brands in terms of breast implant disease?
Sounds strange to me.
So I just had my implants in for 3 months. Started to show signs of Bii. I made the decision to get them removed. I removed them on the 20th of November. I still have severe dry body skin.No oils in my face and inflamed skin.The doctor only took out my implants. Not the capsule itself, will I ever revert back to normal? Or will I be symptomatic my whole life?
Not sure how to contact you, but please know that Dr. Rucker in Eau Claire, Wisconsin is retired. I called his office today 8/10/2022 to inquire about explant and they informed me.
The list has been updated, thank you.
Hello everyone,
I got my 650cc gummy bear silicone implants back in December 2014, and never had any problems besides uncomfortability when working out and sleeping. However, about 2 years ago I started feeling something weird like it’s my hormones and would get these random cold flashes but it would be more rare than anything. Then, in the last year I have been getting cold flashes daily, and feel more discomfort in my joints. That really is it, and before implants I had weird things happen. My theory though even with all this being said is these new symptoms could be the implant finally slowly affecting my body and telling me it’s time. I am looking into explanting and then see how I feel after, to know if that is what is really happening. If I don’t start feeling better, then will look into further testing. Either way I have had these almost 8 years and it could just be time to get them out, I am ready to sleep comfortably and workout without these things getting in the way. One last thing on my left implant I sometimes can feel it, but mine are super soft and natural, so we will see what happens when I finally explant.
Thank you, wish all the best to you ladies.
Is there a section on this site, or link that lists specific skin issues due to BII? I have recently developed dyshidrotic excess – on my hand (this type normally appears on hands & feet). I’ve had implants almost 2 years post mastectomy. I’ve never had any skin issues – this is crazy. Got prescriptions last week for the excema but am wondering if there is a correlation?
Skin rashes is a symptom of BII. It was on the list of symptoms my doctor (Dr Khan) sent me. I also developed dyshydrotic eczema a few years after getting my implants . I have used topical steroid foam twice a day almost every day, plus had systemic and local steroid injections and it would not go away. After explant on July 12, 2022, I am still using the topical steroid, but I am finally seeing the rash go away.
Wow – such great information!! Who created this site??? It’s excellent
First of all, I want to give a big THANK YOU to everyone who has shared their concerns and journeys on this site!!! I have not yet told my family; sisters, adult children and a teenage granddaughter but I will share here now…
I have been following a former classmate of mine who lives in California. She had her breast implant surgery over 25+ years ago and since 2015 has developed several health issues. After seeing a multitude of doctors, she was told there was nothing seriously wrong with her, just allergies and fibromyalgia etc. Her symptoms continued to worsen so she continued research on her own, and finally put the pieces of the puzzle together firmly believing her symptoms were being caused by the implants. She got an appointment with a fabulous surgeon who believed her… Long story short at 73 years of age, last week she had them removed. Success story! Her story awakened me to reality…
I had my implant surgery in April 2007, at 47 years of age. Being very flat chested most of my life especially after having had 3 children and being very slim up until the past year or so. Never noticed too many real issues, and healed fairly quickly but within a couple years developed “arm boobs” which definitely affected my ability to lift weights (I was a gym rat for about 5 years until 2009.) Totally did not like this.
Anyhow since 2019, I have developed health issues, some new and some I had but are now getting worse such as rosacea (my older sisters have this too) and hypothyroidism, diagnosed both in my thirties. My rosacea (or what I thought was) came back full blown. I finally had to see my family doctor’s PA in mid-January. She didn’t think it was rosacea but definitely saw the red-scaling rash on my chin, upper neck areas, and was immediately concerned about infection. I am a natural health practitioner myself and NOT fond of antibiotics but having exhausted all other means, agreed to take them. The rash went away in about five days. I began thinking ‘I’ve developed a food allergy.” In February, almost a month later, same rash occurred only worse. Saw the doctor’s PA again, she prescribed the antibiotic hesitantly (it worked in Jan.) After seven days, the rash was better but not actually healed, although it did fade within the days following. So now, almost a month to the day, I woke this morning with the same FULL-blown rash, same areas but so much worse. I’ve had headaches as well.
I’ve started some extensive research today to learn that I have so many symptoms!!! The rash, swollen lymph glands at random times, numbness in hands (a doctor told me I may have Raynaud’s.) My fingertips down to knuckles turn white and become numb out of nowhere at random times especially if I get cold. Last month my index finger pad started turning BLACK. The only way I can get it to stop is rubbing my hands together gently under warm water. I appear to have an allergy which is getting worse (I’ve had some sinus issues in the past but very minor.) My overall skin condition on my body has declined and does not appear to me to be simply from aging. I get tired easily and also don’t handle stress very well (I thought this to be from the past couple of years.) Since last year I have had dry eyes (Ophthalmologist thought this was from my thyroid condition) and I’ve noticed last month my throat was dry at times when swallowing food. And even though I had my annual eye exam in December, I think my vision is changing again.
So sorry to make this so long! Monday I am calling the plastic surgeons office that originally did my surgery for a consultation. I need to make a list of questions to ask about the explant procedure. I also want a toxicology lab completed. After watching a video from another woman who went through this entire process, her labs were frightening! Even though her implants were not leaking and they also removed the capsules successfully, her body was FULL of plastic type toxins!
I am so on this now. I will keep you all updated as I have my appointments and truly hope to get these implants out a.s.a.p. I know I could encounter some opposition from the surgeon (my original surgeon has retired in 2019.) Another friend from years ago who had implants and chose to remove them was told by her surgeon that “she might not want to do this because afterwards she’s going to look like she has two friend eggs for breasts.” I was like WT..?? Seriously! He was trying to get her to replace them with new ones. LORD HELP US! Some of now know, these are toxic objects no matter what we were told when we opted for this surgery.
I hope and pray for good health for each one of you! GOD bless you all…
6 weeks post op, Enbloc explant and my brain fog is gone!
My joint and back pain is gone. My digestion is so much better. I no longer have the random pains and shooting pains in my chest/heart/lungs like I did before. The only pain today is the tiny bit of lingering aches from where my drains/sutures were. I am still having a tiny bit of allergy symptoms, but next to nothing compared to the radical skin rashes on and around my eyes, face and arms I was enduring. That was for me, one of the worst parts of BII.
I am adjusting to looking down and seeing my feet!! hahaha.. I feel overall healthier, it’s weird and neat at the same time. Doctor Barr has been incredible along with his precious nurse Candi who has been there for every text, call along the way as I wondered if this or that was “normal”
In all honesty, some days I miss having the prettier looking chest, BUT,.. then I consider how great I feel both mentally and physically and am so glad I did it. I use scar cream that is fading my suture lines beautifully. I breast fed 3 little girls many years ago, so the miracle Doctor Barr did to put perk back into my small chest is AMAZING!! Doctor Barr and his entire staff- Candi, Carla, Debbie, Danielle… all of the gals have been so sweet and amazing support- ANGELS!!
Finding this website has been a Godsend. I am explanting in 3 weeks. I have odd rashes pop up on my torso and under my arms, dry eye with occular and facial roscea, joint pain, extreme chronic fatigue, IBS, hair loss, hashimotos thyroid disease, weight gain and on and on I could go. Most of these have come on over the past 3 or 4 years. Also a warning….I had a mammogram and an ultrasound in July 2021 and told them of my breast pain and obvious capsular contracture but they insisted all was good… after I consulted with my surgeon in Jan 2022 I spoke to my PC Dr and told her I still was having paing and I requested they do a MRI. Also thought it would be great info for upcoming surgery. Had it a week ago and found out both are ruptured!!!! Found out that MRIs are only really reliable for implant issues.
Hi everyone. My name is Monica and I live in Chicago. I was once a very active person and in the past 5 years have barely been able to do anything. Last year I began having a burning sensation in the middle of my back and now it’s wrapping around my chest and down my arms. It feels like icy hot plus a heating pad and it’s just unbearable. I also have crippling migraines and occipital neuralgia headaches. My vision has gotten so bad and keeps getting worse; my right eye is like seeing through a film of Vaseline.I no longer wear contacts because my prescription has changed so much in just the last 6 months. I’ve been searching websites for explant surgeons . I’ve narrowed it down to 2-3 but have not made appointments for consultations yet. Does anyone know any in the Chicago area that they have heard great things about? With a lift I honestly don’t care how small my chest is,I can’t wait to be able to breathe again.
Hello Monica,
I am not aware of surgeons in the Chicago area, but I live in Michigan and just had a consult with Dr. Shaher Kahn in Novi, Michigan and he is absolutely amazing to talk with re: BII. I am re-arranging my schedule as we speak to get in for explant asap, I have had my toxic bags inside me for almost 23 years and I am 46 years old with 44/55 of BII symptoms, so thankful to found this group and Dr. Kahn. Hope this referral of doctor helps.
Dr. Kahn has a FB BII group also.
Dr. Lu-Jean Feng (The Lu-Feng Climic) Pepper Pike, Ohio (near Cleveland) https://m.fengclinic.com is a well-known and highly skilled plastic surgeon specializing in explant. She removed mine and did beautiful work. Used the same incision line. She also has a health clinic and counsels you how to get better. Friends drove me and we shared a hotel room for a couple days until my drains were removed. I highly recommend her if you can make the trip. Remember the show “The Waltons”, Erin, the middle girl, became an activist after removing her implants, marymcdonough.com. May God bless and heal you.
I actually have a friend who had her surgery done by Dr. Feng and I understand she is amazing. However, my friend was on a waiting list for over a year. How long did you have to wait from when you made your first phone call until your surgery? I am in terrible shape right now and I’m worried to wait terribly long to have mine removed. Also, and please forgive the question, but can you give me an idea of the cost? Thank you!
My name is Delia 51 years old I live in McAllen Tx I have many symptoms, I had the implants for 7 years. Without money, no insurance. I need help! What can I do? 🙁
Check out National Center for Research Health, Dr. Diana Zuckerberg, website. (Center4research.org).
Delia,
I am in the same boat. I have medicare but explant surgery is cash only. I hear some say that their insurance covers some of it, and maybe it does, but most want cash only. I have learned that if you use a credit card, providing your doctor accepts it, they add on a charge for the credit card fees that is around an additional $2000! That brings the operation from approximately $8000 to $10,000. I am getting a free consultation in Plano in about a month with Dr. Surjit Rai who comes highly recommended. I am driving to his office in order to have a free consultation. I do not think people like us have a choice. We must take out a loan to pay cash and make payments if we want it done. For me, due to all my symptoms getting worse all the time ( I have had my saline implants for 25 years!), I may have no choice but find a way to get a loan. I’m so sorry. I wish I had better news for you. Donna
Go and get the explant surgery in Colombia or Brazil, those countries are the best in plastic surgery and prices are really low (breast implant are less than 2.000 dollars) I bet you could get the explant surgery by about the same price.
Donna: I am in Carrollton and am probably the oldest patient (78) on this site. i had implants placed in 1976, removed in 1992. Symptoms, like others, multiple, painful ruined my life and marriage.i live on Prednisone and pain medication. Many symptoms of Lupus and other autoimmune disorders, as well as frightening and painful neuro symptoms. After all of this time, my breasts are still burning. I would like to locate a PS, who will biopsy breast tissue for residual silicone remaining. Do you know if your Plano surgeon will do this? It angers me to see that these lethal devices are still on the market and promoted as “safe.” My surgeon said they were safe, they would last a lifetime and I would NEVER have to worry about breast cancer!
I’m a breast cancer survivor who had a bilateral mastectomy in 2015. I have Allergan textured implants (the ones recalled in 2019). My health has been seriously declining over the last 2 years. I have 27 of the symptoms on the “poster” linked above. I’ve been seeing a neurologist, ENT, cardiologist, etc for over 2 years. I was hospitalized for severe sepsis (went into septic shock) last year and spent a week in the ICU. I have constant vertigo and intermittent migraines, which my neurologist treats me for. Through all of this, I’ve been working full time. I saw an immunologist a year ago and she did an very deep blood workup… All normal. I went back to her 2 months ago, and she repeated and did even more tests, including genetic testing for over 500 markers… all negative, again. As I’ve been experiencing severe joint pain and body aches, a friend asked me if it could possibly be my implants. I dug up the letter I received from Allergan in 2019, and took it with me to my follow up appointment, and showed it to her. Everything changed that day. She told me everything finally made sense to her, and she “strongly” recommends I get the implants out as soon as possible. That was the day I learned about “BII”. Tonight I found this website, and I’ve been so frustrated, because I can’t keep reading all the information and comments bc I start crying every few minutes and can’t see the words to keep reading. I finally “know” what I’ve been dealing with (as I see myself when I read these comments and other woman’s stories). I just know.
As soon as I can “read” more clearly, I’ll click into the Dr list mentioned above… But does anyone recommend a plastic surgeon with BII experience in the Denver area? I have an appointment with Dr Bateman in November, and Dr Williams in Lone Tree (but not until February… soonest available). I just want these things out so badly, and waiting feels just to get an initial consult is agony. I’ve been struggling just to walk, and make it through my work days. My poor husband has been so worried.
Besides getting to a good doctor and “healing” my immune system, I’m so angry. My decline has put me, my family, and even my coworkers through so much. Are there any law firms in Denver representing women like us?
Thank you for this site, and all of you sharing your stories. 💕
Dr. Linda Huang in Denver
Can implants cause rosacea?
I swear my rosacea started after my breast implants were put in…
Yes rosacea is included in all the skin symptoms.
Thank you for this page, it’s help me to find my doctor. I travel from Netherlands to Hungary to do my Explant and be happy for this because exceeded my expectations. I corrected my symmetry and removed the silicone en bloc, my scar is smaller than I imagined.
My incision is much smaller than I expected also Camila. I feel much better already! 🙂
My doc- Frederic Barr is a perfectionist and leans more natural healing than pushing meds (he provides them but doesn’t push them). His staff was incredible through the entire process.
Please help me. Can breast implants cause severe diarrhea? I read that leaking implants can cause fungal growth in the body and wondered if this could cause the diarrhea? My name is Donna Moore [email protected]. Any help would be greatly appreciated
Yes diarrhea and constipation are symptoms. I saw a lady on Botched who had an explant and BII was acknowledged by the surgeons. Her main symptoms were brain fog and morning diarrhea. Her symptoms stopped following explant. Oddly, no mention was made about enbloc or even capsule removal, or shown during the explant filming.
Catt, I feel your pain, it’s not to late, get them out. Symptoms began for me in 2006. Im 52 now, damn near bed ridden. Thursday these bad boys are Out!
Good lord!!! I knew about BII but never even thought much about it until now. I had my Mentor saline implants in 2002 and still have them. I’m 72 almost 73 so I figured I’d just leave them in for the rest of my life. Now reading the symptoms of BII I’m sure these have caused my health to go downhill for a very long time. I developed hyperthyroidism and Graves Disease in 2010 resulting in the removal of my thyroid and eye surgery to remove the thickened skin behind my eyes. I’m sure I have autoimmune disease and over the past year I’ve developed terrible stomach issues. I’ve recently had all sort of tests which have come back negative except some cellular changes to my esophagus due to acid reflux. I’ve been on medication for this for 2 months and thought I was getting better but now back to feel like crap every day. I’m also depressed and don’t even get out of bed a lot of days. I don’t really know what to do and don’t want to live the rest of my life like this.
Hi Catt, did you have your implants removed?
I’m 71 and I am scheduled this month to have them out!! Hope you are well.
I had mine our yesterday!! Just taking one day at a time the worsr part are these drains hopelly next Tues they willl be out !! I already feel lighter🙌🏻. Praise God!!
I am sitting here in tears reading everything on this site and all the comments. I am convinced I have BII now. I don’t even know what or where to start unraveling this horrible situation. I had implants in for 32 years. They had ruptured but I didn’t experience any severe issues so I didn’t do anything about it until last year. I was having pain in my right shoulder, back, and arm and thought it was probably related (it was). I was also experience the hair loss, joint pain, etc. but wrote that off to aging (I’m 56). I finally decided to explant last year but the surgeon I went to convinced me I would not like the results and that it would required multiple surgeries since I would be left with so much loose skin. He also said I would have to be “banded” for 8 months while my body healed. So, I went ahead and had new implants put in. While I was in the hospital, I developed a rash that he said was from the scrub solution. It was painful like a burn. Then, I developed a horrible rash over the majority of the right side of by body while on the Ceflex antibiotic. I didn’t seek medical attention because I didn’t know what what going on. My body blistered and peeled like a horrible burn. At my check up, I told my surgeon about it and he said it sounded like I had Stevens Johnson Syndrome which is an allergic reaction to the antibiotic. What I didn’t know is that I should DEFINITELY have had medical care. Then, just a few weeks later I developed a horrible case of colitis and ended up in the ER and overnight in the hospital. Since then, 8 months later, I still have frequent bouts of colitis, I cannot eat so many foods now, I have constant diarrhea, joint pain, fatigue, weight gain, and overall feeling like crap. I am so scared I won’t get my life back. This is so awful.
Vicki, Get those implants out!
i have had silicone implants since 1979. i have had constant gastro problems and diarrhea for past 10years. Test after test run, colonoscopy all normal. could my breast implants possibly be causing these problems? i also have thyroid problems changed from hyper to hypo thyroid. Doctors just laugh when you mention BII to them. any suggestions you might have would be appreciated
Yes, all your symptoms are on the BII list. No point in mentioning BII to doctors, except surgeons who acknowledge it on their websites. As I’ve read, all doctors should ask patients if they have any silicone devices in their body, as they ask if you smoke. I’ve seen multiple docs over decades, including functional medicine ones, and NEVER been asked if I had breast implants.
My current doc basically said nothing when I told her about the silicone sheets in my breasts. Just gave me more hormones and an adrenal sup. from urine and saliva results. Been there done that, it won’t do anything.
My Doctors didnt tell me mone were tecalled. So I asked him he said yes. I asked Why they didnt tell me HE WALKED OUT OF THE ROOM. I had them takin out I asked himnto take out my capsules he said they were to my chest. He couldn’t. Now i have 2 lymphnodes swelled my lung and a spot on my liver. Liver doc said autoimmune disease.
I have had my silicone implants since 12/01/ 2000. I have recently been experiencing major pain in my neck shoulder joints. I am unable to sleep or lie down for very long. I have been diagnosed with ischemic colitis in 2018. Should I be looking for a breast implant illness specialist in my area( I live outside of Las Vegas(actually NW Arizona) .
I am a 2x stroke survivor of 25 years( caused by stress). Could someone help me with suggestions on what to do, also where to go? Please help
Hey Catherine,
I am just recently getting tons of
Joint pain also in my neck, shoulders, back, hips, elbows even!!. I learned about BII about 4 months ago. My daughters have been listening to me for the past couple of years complain about all my symptoms .. everything from skin rashes, burning itching eyes- inside and out, brain fog, fatigue, sudden food allergies to things I love and have eaten my entire life, soreness when I work out, radical hair loss all of
The sudden. I literally had been telling my husband I feel like I’m dying daily. My daughters heard about BII on 2 social media platforms and told me mom it sounds like you have BII. Doctors tell me I am healthy and that they have no idea why I am getting these rashes. Been to fam doc, dermatologists, even naturopathic docs. I guess my point here is if you have only one symptom it may or may not be BII- but if you begin looking back since 2000 start trying to think if you have had any other of the symptoms listed.. this is what sent me on my journey recognizing I have BII and knowing these suckers are filled with toxins and things that can kill us- why keep them in. I am Praying for you Catherine. I am getting mine out next month and it cannot come soon enough for me!! If God says I/we are enough, then I need to bask in that; and know/trust this for myself. So sorry you are going through all this crazy too!! If it’s BII you will begin to see patterns over time of
Symptoms you have had. I see
Soo many things that are now totally adding up that align with all the sharing the gals on this page have noted. So grateful for this group and boldness of other women to share their stories to help others (me). I want to do the same! Ps.. docs are so beyond busy – wait time across the US is 3-6 months so book in advance should you decide you want them out. I live in AZ but going to Florida to have mine removed.
UPDATE- I got my explant last Wednesday by incredible Doctor Frederic M. Barr. -he and his office staff have been awesome!! I am doing fine. Feel free to ask any questions. Many symptoms have completely disappeared already. Looking so forward to seeing how the final healing looks over the next 4 months. Sooo glad I did it!! Implants are making so many of us sick!! We are enough naturally!! 🥰❤️🙌🏼🥰🙏🏼🙏🏼
I have saline implants can they cause the same problems
Hi Pam,
Yes. The saline implants also cause problems. The shell of the implant is made of silicone. The silicone will leak into your body and begin causing illness or autoimmune problems. I have saline implants as well. Have had them for 18 years. Been getting sicker and sicker for the past ten years. I’m planning to have mine removed asap. This is what opened my eyes to my illness’s. Good luck and God Bless.
Cheryl,
I’m teary eyed reading this. I just prayed for you!
Thank you so much for sharing this! I feel like I am going crazy with my symptoms.
I also just learned about breast implant illness a few months ago, thanks to my two adult daughter’s finding the page on facebook. I have almost all of the symptoms listed above. I am going to a consult with a doc today- I am in excruciating pain and like you I have excused every symptom attributing them to getting older (54). I dont even look like myself in the mirror my eyes feel like sand is in them, the skin around my eyes feels like a combo of acid, sting and itch like nothing I have ever had.. and then the rashes that keep showing up all over my arms, neck are so radical!! I am so sorry you are going through this. My hubs is struggling with me taking these out, but geesh I want to live and be the healthy girl i used to be. I no longer have desire to work out- SOOOO NOT ME! I too have gained weight that no matter what I cannot lose, my hair is falling out (more than I can afford). Ughhhh.. God is the master physician He will heal you! … and me too!! God bless you friend.
Attachment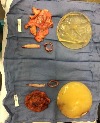
I am so thankful that I found your site. I am 6 weeks post explant and extremely ill. I had the original Owens Corning implants for 34 years. I never thought my implants were causing my body any harm. I was that ostrich with my head in the sand. I have an antibiotic resistant bacteria that we are fighting that became active upon removal. I’m so glad we (my husband and I) found a surgeon who has been extremely thorough with the explant and also very sympathetic. After surgery Dr Eby brought in an infectious disease doctor to help with my issues. Dr Patricia Eby of Memphis is my surgeon and she said my implants were the worst she had ever seen and that she unleashed Pandora’s Box with the first incision. My surgery took 5 1/2 hours for explant, cleaning, and reconstruction without implants. I was worried I would look mutilated but my reconstruction is amazing and only needs to heal. I am in extreme pain all over my body. I had no idea the amount of toxins that have been destroying my health and my life. My blood work last week showed lupus titers but we’re hoping that is from the extreme amount of infection and inflammation we are battling from the surgery. I didn’t know anything about BII until 3 months ago. Yes, I obviously ignored the warnings of my body. Dr Eby and I feel like my implants were ruptured about 10 years ago. I remember the mammogram (didn’t know how bad they are for implants) and the reactions afterwards when my first symptoms appeared. Three days after the mammogram I looked like I had a bad burn and rash on my chest. The mammogram center said I must have had lotion residue on my chest. (SMH) Now I feel like an idiot for ignoring my body’s pleas for help. I have had for last 10 years these symptoms: thyroid issues, thin hair, weight gain, severe respiratory issues, chest pain, joint pain, wheezing, pneumonia, fatigue, brain fog, memory loss, Epstein Barr Syndrome, Bells Palsy, shingles……. I have attached the photo of the implants, casings, and scar tissue post surgery. My husband has the implants but I haven’t had the stomach to look at them yet. Right now I’m dealing with recovery from the surgery and all the side effects of the antibiotics. I plan to be an advocate to help others learn of the dangers of implants and to convince women to stay away from them.
Thank you so much for your sharing, I had my implants from about 12 years. I also had a massive prolapse disk and chronic pain 3 years ago… since then everything went down the hill… chronic fatigue, join pain etc… I thought was from that event but now I’m thinking must because of this?
Can I ask what makes you think was this causing all the damaging?
Also how you found a good Doctor? I live in Nz and I’m feel so afraid of the quality of doctors here 🙁
Cheryl, Your story sounds very familiar….Thursday I’m explanting by Dr. Shaher in Michigan. It is a four hour drive from Pittsburgh, Pa. Considering my condition now, that drive is nothing. I’m a bit anxious but so ready to begin a new chapter. Symptoms start out slowly, with no real treatment. Year after year, it’s now chronic fatigue and pain. Ladies, if you are reading these comments and have any symptoms, please consider BII and get them explanted. I’ve been sick for over 15 years. I still have a chance at life.
I just want to update folks on what I learned about some of the doctors on this list when I called them for consultations:
Dr. Stanwix Henrico, VA Phone consultation fee of 50.00 to put you on a phone list. 4-6 weeks is about the average time to talk to him. then it’s a 250.00 dollar consultation fee that will go toward surgery if you decide to go with him then they book out surgery3-4 months. Fees: Explant and en-bloc$8-10 thousand with lift around 14,000. done in a surgical center. he won’t deal with insurance but will give you the paperwork needed.
Dr. De Rosa, Detroit Michigan( only does explants and en-bloc now) 6-7 thousand and around 3 grand more to include lift. does both surgeries in a hospital and surgical center, does drain most 5 days, She is special because she had implants that she had to remove due to illness as well! I believe a video consult is about 100.00 she is booked out till March for surgeries, Fantastic assistant Jim!
Dr.Bruinewicz, Doylestown PA and NJ. 150.00 virtual consulting or in person. does hospital surgeries, not sure of his cost will know more in November.
Dr. David Passaretti. 250.00 consultation, has his own surgical center. Does not do drains. $18,250 for explant, en-bloc, and lift with faculty fee and anesthesia, they gave me a discount of 4500.00 I am not sure why and they have someone that specializes in insurance so I don’t know yet if I will get it. they will get back to me. Fantastic assistant Caunie!
Dr. Alperovich New Haven CT. I needed a referral from my Primary or some other doctor. then they called to make an appointment he is booked out to July 2022
Dr. Black, Metairie, LA, Does not charge a consulting fee, even called me personally but unfortunately, we played phone tag and I haven’t been able to chat with her yet but she has raving reviews on the explant and en-bloc she does.
I hope this helps anyone out there, what a lot of work this is. thank you to whoever started this page!
Excellent summary & feedback, thank you!
Beth, thank you! I am looking for a surgeon now, and the three I’ve been looking at are not on your list. Are the doctors on your list the ones that you recommend the most?
Thank you Beth! I am desperately trying to find an en bloc doc ASAP! All the ones I call are backed up until Feb March next year. Appreciate your thoughtfulness!! So grateful 2 of my daughters told me of FB page.
I had my explant 13 months ago, both implants were ruptured. I am now having severe swelling under my armpits and a lot of inflammation.
Thanks for sharing… just a question, I’m new on this. So you are not feeling better? I’m confuse 🙁
Karen, Sounds like you have blocked lymph nodes in your armpits and maybe all the silicone wasn’t removed. I have recently seen a surgeon in Australia about breast fat transfer, who also specializes in enbloc implant and capsule removal. He also knows about BII. All explants are photographed for the patient and capsules sent to pathology.
I had explant in 2012. I asked for enbloc and photos. After explant I was told my capsules were paper thin. The surgeon said nothing about removal of them and there were no photos of them, so I figured that was his way of saying he left them in because it was too difficult to remove paper thin capsules. To this day my health hasn’t improved. The current surgeon ordered a special MRI that shows silicone, called a Silicone Face MRI. I have sheets of silicone over the muscle (where the implants were) in each breast, 3.5cm x 6 cm x .3mm in thickness. No silicone in armpit lymph nodes. Doc said he could remove them.
Did your surgeon say he removed all the silicone? Without the MRI, you wouldn’t know for sure.
My name is Amy, I live in Portland Oregon. I have had almost all of the BII symptoms for the last year and a half .Debilitating daily pain that has prevented me from doing my work. I have had depression, hair loss, brain fog, dizziness. My primary symptom has been extreme daily muscle pain isolated to my right upper back and shoulder. I have been to / 20 doctors and practitioners without a treatment plan or a diagnosis. My implants were put in 6 years ago. I am looking for a Doctor that may be able to explain things further and or possibly do an exam to my breast implants. Does anyone have advice for this type of doctor in Portland Oregon?
Hi Amy. Did you browse the list of explant surgeons in your area? If not, I highly recommend you join one of the many Facebook groups for breast implant illness. You can post any question and people are very supportive and helpful. Goo luck
So happy to have found this site! I am a breast cancer survivor of 15 years. Abdominal tram flap reconstruction along with saline implants. Approximately 3 years ago, I started developing numbness & tingling in my feet that has progressed to arms, fingers & face. Very painful! Multiple sclerosis has been ruled out. Now I’m on a quest to have these implants removed. I live in Omaha, Nebraska & I was wondering if there are any surgeons taking BII seriously in my area? I have an appointment with a plastic surgeon but not until June. Dr. Honz with Methodist physician clinic is where I have started my search.
I’m 34 has my saline implants 10+ years ago. Two years ago I woke up and had high pulse with extreme anxiety, by doctor said I have sinus tachycardia which is strange. Now my symptoms are getting worse I’ve had lots of blood work done I had a colonoscopy thinking I had colon cancer. I had ct scan of pelvic and ovary which were all good. I’ve been to the doctor so much to my doctor said there’s nothing more I can do and to go see a psychiatrist. Iam so I’ll I am convinced I have inflammatory breast cancer! I’m super weak have digestive issues muscle cramps severe depression and anxiety, high pulse, neck pain. I literally feel like Iam dying. I’m scared to go to bed at night afraid I won’t wake up. And cry everyday looking at my daughter and thinking what she will do if I die being I’m the only one she has. I have vision problems, hearing loss. Neck pain. The list goes on and on. Now my right boob is inflamed and itches. And is tender. Is it cancer or breast implant illness? I can’t get In till the 22nd of this month and I need some answers. Anyone have similar problems?
I am new to this site and have had a year of specialists, MRI’s brain scans, etc.., with doctors telling me i’m causing it and have anxiety….which is ridiculous! I used to be a fitness instructor and have been active and healthy my whole adult life! I think the BII is the root cause, I have felt like I’m dying, numbness in hands & feet, scratchy throat choking feeling, insane levels of anxiety, chronic fatigue, vision problems, ringing in my ear…, etc, etc. (so many things on the symptoms list) Find a reputable surgeon who can do an ENBLOCK EXPLANT CAPSELECTOMy, they have to remove the ENTIRE capsule or you will likely have to have the surgery redone. Italked with a lady locally who just had the surgery done. I am in the early phase of getting a consult and then praying I can find someone to help. I have never experienced anything like this in my life. Hang in there and good luck!!
Jenna,
I am going through much of what you are and have been scheduling consults with docs to see who can get me in the soonest for explant. The 2 docs I have met with both confirmed I have all the typical symptoms and a precious gal named Laura who was on the Oprah show back in the 90’s has taken me under her wing. She went through all of this and now video tapes for a doc in Chicago all the explant surgeries. She sees your type of symptoms all day long.. so sorry you are going through this too!! Many docs will do free phone consults. I found a couple in TX and Florida. I just need to find one sooner than March or next year. I’m going crazy from rashes and feeling like I’m dying.
Hi April. My name is Monica and I live in Chicago and am searching for explant surgeons. Do you know the name of the doctors that Laura video tapes for? I have only found one in the city and 2 a bit further out. Thank You
Hi Mayra,
How are you? What symptoms do you have?
I think it is pretty real too. My family doctor laughed at me when I ask her about BII… The Emergency doctor also. What can we do? They don’t want to believe. Their choice. Althouh so not fair for any of us. For the last month, I came to term with all this. I want to come back to the person I was before all this. At least I will do my best. And explant is the only thing that will help. Cause at this point I am scared to have this inside me. It is my body and only I can know if/that something is wrong. And trust me something is wrong. I will have surgery October 1st. En bloc or total capsulectomy.
I was worried about my husband and everyone else for that matter. What will they think? Will he still love me? And truth to be told it is their buisness not mine. So I am choosing myself cause I want to be healthy for my kids. The amount of growth I am gaining from this is substantial and I believe we all have something to learn. For me is to stop living for others. This mess has force me to listen to myself and make that big decision all by myself.
If you feel something is wrong listen to your body, please. Cause tomorrow another symptom will appear and then another. And it is so easy tp blame that symptom on something else. But how is it possible to have that many thnigs happening one after the other…?
Hope you are good Mayra. Happy to chat whenever, if you need.
Unbelievable information. Your team is a god send. I am on the path to fixing my wife. She has had an immense amount of chronic chest pain (costochondritis) and fatigue. She has gone from an active, working out 5 times a week to not wanting to go for walks. Thanks for the guidance on what steps to take. I am making an appointment for consultation right now.
Hi Mike. I have so much chest pain and it hurst when I move my arms as well. I had a chest X-ray but came back normal. I thought about having costochondrities, but think it would have shown on the X-ray?! How was your wife diagnosed with it?
I am here with you. NOT BEING DIAGNOSED is madness. I to have so many symptoms. My biggest one being the damn pain. Every day, all day. Let’s stay in touch? I need support so badly.
Hi there,
This page has been godsend. Thank you to everyone who post cause I found out so many things.
I have my implants not even a year (got them last October). 2 weeks after I developed bad acne (face, chest, arms) that wont go away. It is a nightmare cause I am now so self concious and far from being confident (Which I tought I would after getting implants…) I have now severe anxiety and althought I never suffer from depression some days I feel so horrible that I can’t stop saying to my husband that I don’t know what is happening to me and that I don’t recognize myself on those days. Memory loss, brain fog that keeps me from working as much as I used to. I stoped doing things I used to love so much like training. Worse is most days I feel like I would have after a crazy training session. How can that be? My legs are weak when I walk. I can’t catch my breathe after going up the stairs and so on. For the last month I am numb in one feet and sometimes arms too. I gained weight. I am dizzy. I have heart palpitation and the list goes on. Passed so many tests, MRI, Blood work. Doctor tought I had a stroke that was causing numbness and memory loss, confusion. I was so scared.
I have come to the conclusion that I need to take my implants out. But what is hard is my husband doesn’t really believe in BII. He thinks I am so stressed about my implants that I create the symptoms. Plus he loves my breasts so much better now. He doesn’t really want me to take them out. He says I should wait and see cause what if I take them out and I still feel the same. He has a point there and that what’s makes me so scared. This is even more confusing to me cause I will have 3 bad days in a row and sometines 2 weeks of better days, except acne never leaves me… As I read all the comments though I understand that it is a matter of time before I have more bad days than good days.
My question:
I met an amazing, caring explant surgeon this week that I found on this website. He explained everything to me and confirm that it is BII. He said I should have them removed. He explained that he can do a total capsulectomy but can’t guaranty the En bloc as sometimes the tissue is too thin that he has to leave it. Is that OK? Will I still have toxicity in my body if he can’t remove everything? I appreciate that he was honest and said that in his practice it is 50/50 that he actually can do an Enbloc.
To all ladies the have done an explant, did the surgeons removed all of the scar tissue?
Sorry my post is so long. i feel terribly alone in all this as I am trying to gather all the information to make the best decision.
Hugs to you all
Hi, I had my surgery 6 months ago, but I started feeling symptoms of BII, I went to my doctor and she said BII is bullshit, I don’t think so, I think this shit is real, what did you do Mary?
I have all of those symptoms as well. What symptoms are different compared to inflammatory breast cancer and breast implant illness?
Hi Mary,
You said acne on face and arms but not a rash huh? I have much of what you described. The itching burning is like constant tormenting. I look on motor and my eyes are so swollen everyday, I always look so unhealthy but had bloodwork EVERYTHING checked and no one know what’s causing my symptoms..
Obtem foi o neu dia de sorte, tirei com 10 anos, vi a capsula e por dentro, ja estava um horror. Tirei com o Dr Paulo Costa no Hospital da Prelada aqui no Porto. Um anjo que uma cliente Italiana recomendou. Força ai minhas senhoras, é urgente.
Mary,
You are my twin in so many ways!! My hubs is REALLY STRUGGLING with me removing mine also.
I am seeing my first consult today. I feel EXACTLY AS YOU DO. You are Not Crazy or making your own symptoms. I told my husband I want to feel good and healthy again and get back to all the living I am used to doing before the last two years.
I have said the same “what is happening to me?” “I feel like I’m dying.” ” I look so swollen and old.” I am someone who loves to workout. I am getting mine out as soon as they can fit me in. Here in AZ the docs on the list are backlogged until middle of next year- insane!! Praying for a cancellation- I feel like I’m in a race against the clock reading everyone’s posts. My doc in So Cal who put these suckers in, doesn’t even recognize breast implant illness and yet “he will do a complete capsulectomy” Imagine that!! GRHHHHHHH!! AND, HES ON DISCOVERY HEALTH ALL THE TIME FOR HOW GREAT HIS IMPLANT WORK IS. SMH!!
Praying for you to know that you are enough big boobs or not. Praying for your hubs to come around too. Mine finally is, after some long convos reversing circumstances. ; )
Has anyone had any experience with a Dr. John Pierce in Scottsdale, AZ? He is recommended on this site but I have not found any reviews or comments. I will need to have explant surgery within the next two or three months. My implants are 25 years old saline and I think they are causing my issues that have been occurring over the past 10 years. More recently one of my implants is sticking out the side of my chest wall, and I have terrible fibromylgia shoulders and feet. Any help for surgeons in the Phoenix are would be appreciated. I am trying to get this paid under medicare as i am 67.
Hello,
Did you have the surgery with him? How are you today?
Hello!
This June 9 I will have surgery to remove my implants with Dr. Rubinstein in Thousand Oaks CA. I find his name on your website.
I wanted to ask if you could recommend me which pathology test to take after surgery? I have capsular contracture for sure. Thanks for all the information you’re providing. It was a relief knowing that all the symptoms I’m having are for a reason!
20 Years Lost…
Just beginning testing, as Explant was over a year ago, and I am still having many symptoms of Bii. My internists said my Autoimmune system may have been activated, but didn’t deactivate after surgury. Also, my Board Certified Plastic Surgeon refused to give me my Mentor implants after Ex- Plant. Don’t make same mistake is anyone is considering Explant. GET your implants back.
This is an overview of my 20 years of HELL because of Mentor Implants…and my request to my Internist for Lab Tests.
Attention: Dr. *********, March 4, 2021
Patient: *** ******
DOB: ********
* Full Diagnostic Panel to access for Bacteria, Viral, or Autoimmune Complications (silicone toxicity, heavy metal toxicity, chemical toxicity, and bio-toxicity currently in my system). Un-diagnosed pea sized to marble sized invisible firm, very painful nodules located on arms, torso, buttocks, thighs, calves. (not visible to the eye).
This is a rough Chronological Overview of onset of my “Lumps/Marbles” painful nodules/lumps ( various sizes), entire body front & back
0/0/1997 – Implants (breast): McGhan Brand; Surgeon, Dr. *******
09/1998 – Implants (breast): Mentor Brand; Surgeon, Dr. *******
12/1999 – Nodules (marbles/pea like lumps) noticed in December 1999, and have since multiplied over entire body. All are very painful and would be described as ‘Invisible Bruised Lumps’. Cannot be seen on dermis, and only with use of a lubricant and pressure placed on the lump can anyone else feel them. Located on arms, torso, back, buttocks, thighs, calves. Very painful.
2002: Diagnosed with Radiculopathy, severe back pain, 5-7 Strokes in each eye, as diagnosed by ************* , cause unknown.
2015-2020 – Began to consider having Explant as I had been experiencing many odd health problems, most of which were unable to be diagnosed. Chart below will reference some of the symptoms I have had since 1999 while having implants, however all symptoms have NOT dissipated even after the Explant on February 26, 2020.
02/20 – Explant of Mentor Implants.; Surgeon, Dr. ******* . Dr. ******* stated to my husband, that the removed implants were not damaged, and appeared the saline was clear. He did not send the implants off for pathology as the fluid was ‘clear’. Neither my husband Bob, nor I saw implants post surgery. Nor, would the Dr. Give me the implants, after Explant.
Severe Complication Onset approx dates:
09/99 – Joints were so stiff and painful, I could barely walk straight, and had difficulty getting in and out of a large SUV.
December 1999: Discovered a very painful nodule on upper right backside of arm 2-3″ above my elbow was a very sore sub-dermal lump. Realized it was there because my Mother tapped me to get me attention, and it hurt badly. There was no discoloration of the skin; lump was not raised above dermis; not visual in any way. Since that time, more and more lumps have continued to form under dermis all over body. It is not a ‘flare-up’ of any sort, as they are always there, and always very painful to the touch. Some lumps can even become painful just by normal body movements such as walking , leaning on an object, or just walking. Even now, 1 year Explant surgery, the lumps are still there, still very painful, and I still have many of the symptoms as listed below.
03/2019 – in Spring, began to experience joint pain, worsening to the point I could barely walk or get out of an SUV. Prior to then, had experienced no severe joint pain, and I could do yoga, or bicycle.
02/20 – Over the 20 years I had developed 34 of the 69 symptoms of Bii since having placement of the Mentor Implants in 1999. Beginning in August of 1999, my joints were so stiff and painful, I could barely walk straight, and had difficulty getting in and out of a large SUV.
03/17/21, I am still having some of these symptoms, plus additional symptoms, even 1 year after post-Explant. Including weird rash on boobs, stomach, that cannot be felt, only seen visibly. I did not have implants put back, nor did I have lift.
I still have many of the following symptoms, even after Explant.
YES = Still have symptoms after Explant
NO = Disappeared after Explant
SOME = ON/Off Flare up’s
1. Fatigue or chronic fatigue (YES)
2. Cognitive dysfunction (brain fog, difficulty concentrating, word retrieval, memory loss) (YES)
3. Muscle aches, pain, and weakness (YES)
4. Severe joint pain and soreness (SOME)
5. Temperature intolerance (YES)
6. Low libido (YES)
7. Ringing in the ears (YES)
8. Heart palpitations (SOME)
9. Night sweats (YES)
10. Skin rashes (YES)
11. Insomnia (NO)
12. Estrogen/progesterone imbalance, diminishing hormones, or early menopause (YES)
13. Swollen and tender lymph nodes in the breast area, underarms, throat, neck, or groin (NO)
14. Tingling or numbness in the arms and legs (YES)
15. Burning pain around the chest wall or breasts (I have had ‘striking lighting type pain’) (YES)
16. Vertigo (YES)
17. Fevers (YES)
18. Dehydration (YES)
19. Chronic neck and back pain (YES)
20. Nail changes (cracking, splitting, slow growth, etc.) (YES)
21. Decline in vision or vision disturbances (YES)
22. Liver and kidney dysfunction (YES)
23. Gastrointestinal and digestive issues (YES)
24. Sudden food intolerances and allergies (SOME)
25. Smell or chemical sensitivities (YES)
26. New or persistent infections –
viral, bacterial, and/or fungal (candida) (YES)
27. Reoccurring sinus, yeast, and UTI infections (YES)
28. Chronic inflammation (YES) (YES) & (YES)
29. Headaches, dizziness, and migraines (YES)
30. Mood swings, emotional instability (YES)
31. Anxiety, panic attacks (YES) & (YES)
32. Suicidal thoughts (SOME, RARE NOW)
33. Depression (YES)
34. Symptoms or diagnosis of fibromyalgia (YES) (YES) & (YES)
Autoimmune Symptoms experienced:
1. No symptoms or diagnosis of EBV (- maternal uncle died of Epstein-Barre in 1998)
2. Rheumatoid Arthritis Symptoms – Yes. I have had symptoms, severe joint pain in hips and knees.
3. Lupus Symptoms – I have symptoms. (- paternal grandmother died at 37 yrs due to Lupus, Reynaud’s, and Kidney failure in 1952)
4. Sjörgen’s syndrome ???
5. Raynaud’s syndrome. No symptoms. {My paternal grandmother died at 37 yrs due to Lupus, Reynaud’s, and Kidney failure in 1952)
8. Scleroderma – I have had symptoms. Hip, knee, ankles.
9. Multiple Sclerosis – I have had symptoms, Had 2 spinal taps in 2003/04, negative results at that time; Provider: Dr. *******, Neurologist
Hello,
My heart truly goes out to you and the pain you are suffering. Would you be so kind as to tell us your explanting surgeon?
Thank you and God bless!
Roe, Dear God!!!! Dear God!!! You are me to an exact!! I first had silicone implants in 1990. I had them removed in 2001 and my doctor would not give me the implants after explant. Also I have had lymph node biopsy and at least two of my lymph nodes were full of silicone. I have little pea size nodules all over my right side where my breast implants were and no one can seem to tell me what they are, except, Calcification’s. I stupidly put saline implants in 2010. And had them removed October 2020. Was a VERY active person, cleaned homes for a living, I never ever had a bladder infection in my life until four months after getting these evil devices in my body. I have suffered from Bladder pain/bleeding with clots for almost 30 years now. Going from urologist to urologist to tell me sometimes its a bladder infection and sometimes they have no answer as to why I “feel like I am pushing a baby out of my urethra”, with only blood and clots coming out! To this day I will have an episode of this once to twice a year. And the pain is horrific. Also, I have had night sweats for almost as long, with Dr. after Dr. telling me for 30 years I must be “going thru the change”. Had a partial hysterectomy in 1997!! I am fatigued beyond description and now have an in-home daycare, and I want to just cry at times as I have had insomnia now for going on 2 years! I have a cyst on my kidney. I hurt with joint pain all over, this past two years. So bad that I cry at night in pain. All Rheumatoid tests come back negative! Dr. says she thinks I have Fibromyalgia. Very bad cold/heat intolerance. Heart palpitations. I had a horrible smokers cough, (I do not smoke) that went away 1 week after explant in 2020. My chest at times still feels heavy and almost like someone is hugging me at times in my breasts. The list goes on and on. I have mentioned BII many times to many doctors and all I get is the “Side Eye” or “Absolutely does not have anything to do with your symptoms”. My second explant doctor even told me, “Mary, I have many patients that miraculously feel better psychologically in their heads and their symptoms go away”!!!!!!!! I mixed my words up now, and have terrible brain fog. I just want to cry and sometimes almost die as I am normally a very upbeat, happy, joyful person.
I just had my explant. Before implants I had some mild allergies but otherwise I was completely healthy, very fit. I was assured that my implants were safe after doing my “research” and consulting with multiple surgeons. Nursed two children and had large breasts when I was younger, just hoped to fill out my breasts to their previous volume. Mentor 600cc smooth silicone back in 2016. Asked about BII, risks at implant, told it was safe and not to worry. Started feeling worse after implant, blamed the large implant for the muscle soreness, weight gain and fatigue. Second surgery 2017 to fix placement. Just got worse, started having intense joint pain, unable to run, lift or work out at all. Intense headaches, asthma, difficulty breathing, rashes, anxiety, weird heart flutter/panic symptoms I had never experienced out of no where. Tipping point for me was this last summer I noticed i started breaking out in terrible hives/rash in the sun and could not even hike or be lightly active due to joint pain and fatigue. I am really hoping my recent explant will reverse this. So frustrated that I implanted at all. I was so healthy before and implants stole 6 years of my life.
Hi Kimberly,
What surgeon did you see for explant?
Oh my gosh. I just went over the symptoms list. I don’t usually get to excited when I see symptom lists because I have been disagnised with so much and I was a nurse so I know diseased “Bodies” tend to exhibit a lot of the same symptoms. Well, I have been diagnosed with mast cell activation and it’s the very reason I am on disability because it is debilitating! I also have dysautonomia and fibromyalgia. All listed at the bottom of the symptoms list. I am completely blown away. My mother is very healthy. My aunts are all healthy. My mom is the youngest at 77. And these ladies have more energy than I do. This is crazy to me. I feel angry. Like I have lostbYEARS due to my implants. My surgeon assured me they were safe.
Hi, I still have my saline implants. I am 48 and on disability. I was very healthy and used to work out daily and had a lot of energy. Now I find it a real challenge to be active. I had some issues prior to implants but since having them my quality of life has drastically gone down hill. I do not believe they are the source of ALL of my issues but I do believe they have severely crippled my body’s ability to handle even the smallest obstacle. I had them in 2001. In Michigan. Novi I believe. I would like them removed and am trying to raise enough money to do so. Not being able to work has made it difficult. I was a nurse. I had no idea back then that implants could cause these problems. Does anyone know if Medicare covers any part of the surgery?
Hi Phoebe,
Women have been able to get Medicare to cover surgery, see Insurance and National Center for Health Research: Will Medicare Pay to Remove My Breast Implants?
Hi there, I do not know how to post my own thread so I’ll reply here I that’s OK.
I see a surgeon name in here. Dr. Arturo Valdez in cancun Mexico for explants with lift. The prices quoted are out dated . The costs now include pre surgery covid protocols such as Lung CT scan and covid testing . The average cost now for explant with lift with Dr. Valdez is now approx $6,000 USD . I hope this helps.
Hi phoebe, I am 67 and also need explant surgery. Medicare will pay for explant surgery as shown below from the Medicare website.
Medicare
2. Removal of BREAST IMPLANTs
For a patient who has had an implant(s) placed for reconstructive or cosmetic purposes, Medicare considers treatment of any one or more of the following conditions to be medically necessary:
• Broken or failed implant
• Infection
• Implant extrusion
• Siliconoma or granuloma
• Interference with diagnosis of breast cancer
• Painful capsular contracture with disfigurement
Pain and capsular contracture are key words to mention she said.
Need to have done is a EN BLOC/TOTAL CAPSULECTOMY The capsule will continue to grow if not removed. It can grow around the lungs, heart, ribs, plus the contaminates that are in the capsule.
• A doctor can be a Medicare-enrolled provider, a non-participating provider, or an opt-out provider.
The Trouble is finding a doctor that will accept or even fill out a small amount of paperwork so that you can submit the necessary paperwork to Medicare. You need to realize that most implant surgeons are greedy, primadonnas that are good at selling implants, but short on going the extra step to do anything except collect money. Depending on the area of the country it may be harder to find someone. In the Phoenix area the only doctor that will take Medicare is a non-board certified plastic surgeon who I would not trust. If you are in Ohio I would check out primadonna Midwest Breast & Aesthetic Surgery. They have youtube discussions, etc.
Of all the symptoms I have, the one that is THE MOST HORRIFYING is HAIR LOSS. I used to have so much thick hair, but now, I can see my scalp so effing easily. I am scared. I had people telling me that this is normal to lose hair after anesthesia, yet I didn’t started to lose them right after I had my rhinoplasty to fix a deviated septum. Several years ago, when I had the surgery, right after it, I lost so much hair it was horrible, and from that point onward, it was like my metabolism did its best to cope until it just gave up. It has given up now. I am looking for a surgeon but finding one that will accept to do the surgery with the cost taken care by insurrance is difficult because they won’t see the money as fast as if you pay them beforehand. Does any of you had that symptom ? And more importantly, DID YOUR LOST MANE GREW BACK AFTER EXPLANT ? I am even considering doing PIP to boost their regrowth after getting ride of those rotten pieces of curse. Please guys, if you have updates to share, take a few time to do so. Happy new year to you all !
Attachment
I was diagnosed with Multiple Sclerosis (MS) in October 2011, at the age of 44. I woke up one morning with numbness in my lower back and legs, I couldn’t feel my feet touching the floor. I saw my doctor and had an MRI to see if I had a disc problem, it was negative and she told me she feared MS. I was sent to a neurologist, had two more MRIs, and was told that night that I have four lesions on my spine MS. I tried every shots available but nothing worked. In 2015, my neurologist and I decided to go with natural treatment and was introduced to Mayaka Natural Clinic natural organic MS Herbal formula, i had a total decline of symptoms with this treatment, the numbness, terrible back pains, stiffness, body weakness, double vision, depression and others has subsided. This treatment is a breakthrough for all suffering from Multiple sclerosis, i am strong again!
Hi Debbbie, I too was diagnosed about 2 years after having my implants. I am looking into this treatment as I hate pills and drugs. thank you for this exciting input. I had my explant in Aug.2020. Most BII is gone,but I still have a few. I havent been to my neurologist since explant.
has anyone had eustachian tube dysfunction from BII?
Yes! My ears fill with fluid and it really effects my hearing. I’ve had multiple ear/sinus infections since getting my implants. This has been a huge problem for me. I’ve been very close to having my ear drum rupture multiple times.
My left ear feels as it is clogged up. We’ve made tests and they all came negative : I can ear perfectly and my sinus are well shaped so it’s not coming from that. I cannot wait to get ride of my implants !
I have had this and I never thought about the connection. I used to get muffled hearing when I would eat salt. It still happens occasionally.
I’m Estella San Diego. I got my second pair of implants Oct 2 2020. Problem after problem. I have multiparasites. I’ve been going to the ER and the dr. For about 3 weeks. No one believes me. They think I’m on meth. O one would give me the meds for parasites and they still haven’t figured it out I did. I have open wounds on my arms and legs. Parasites trying to come out of my skin. The Dr. said it was healing so I accepted that. It won’t heal at all. Need them out ASAP. I even went to him 1 week ago one is healed the other is bad.
Go to NSC24 website and look up, Immunecleanse. That is formulated to take out parasites and fungus.
I love this product. It will work.
Hope this helps.
Does anyone know of a surgeon who will perform Explant for ruptured silicone implants when patient is in her 1st or 2nd trimester? Patient was planning on getting pregnant after Explant surgery but in fact got pregnant before – she’s scared about leaving her leaking implants in through pregnancy (MRI confirmed they are leaking) any advice? Does anyone know of any surgeons in the LA area that will perform an emergency Explant? Thank you….
Has anyone used or have any feedback on Dr. Maurine Waterhouse in Louisville, KY??
Hello,
What a great resource for patients! I’m the medical assistant to Tim Sayed M.D. in La Jolla, Ca. Dr. Sayed has been doing several en bloc surgeries per week for the past two years. We would love to be a part of this website. Can you please advise us on who we can contact to list Dr. Sayed’s office? We are here for the BII community.
Kindly,
Dannielle Crouch
Hi Dannielle,
Dr. Sayed was added in July 2019 to the Explant Surgeons list and is currently on it. Thank you for supporting the BII community!
Hello, and thank you for helping educate people about Breast Implant Illness. I would like to know why Dr. Shaher Khan isn’t included in the top portion of your Surgeons List. I would also like for information about him in your Florida section to be update. Dr. Khan not only “listens” to info about BII, he literally “preaches” about it. I doubt anyone knows and shares more about BII and has more experience doing En Bloc Total Capsulectomy than Dr. Khan, Dr. Chun and Dr. Feng. Dr. Khan even has a Public Service Announcement about BII airing on TV in Michigan. Dr. Khan frequently posts videos of surgery and photos of implants with and without the capsules on his FB page and YouTube Channel. He shows photos of patients’ chests to prove there is no residual capsule. He blocks out 4 hours for each surgery and he does about 300 en blocs per year. He has only done implants once in his career, and that was a post-mastectomy patient and it was for his boards. That’s it! I doubt any other plastic surgeon can say that. Dr. Khan sends tissue/fluids to a high-quality lab after every explant. He returns the implants to patients. He is truly amazing. I’m a patient – not an employee. How can I help get his name moved up near the top of your list with Dr. Chun, etc. and how can I help get the info updated that is included with his listing for your Florida section?
OH MY!! I have no idea why I typed “Florida” above. Dr. Shaher Khan is in Novi, Michigan – outside of Detroit. Is it possible to correct that? I’m so embarrassed.
I THINK THAT ITS ALSO IMPORTANT TO DO HYPERBARIC OXYGEN BEFORE AND AFTER EXPLANT. THIS WILL HELP HEAL TISSUES IN THE BODY. WE NEED OXYGEN TO SURVIVE
Attachment
Dear organizers,
I see my name on your website as one of the explant surgeons in UAE. The information is not complete and I would like to give you more information if you can contact me.
Middle East:
Dr. Ashok Govila – Dubai, UAE
Kindly add some details as below
I do enblock capsulectomy with implant removal and give all pictures and videos of the surgery along with the implants to the patients. Any liquid collected is sent for CD-30 test. Histopathology report is handed over in 2 weeks time
Hi Dr. Ashok,
Thank you for the additional information. It has all been updated except for adding en bloc. Three sets of en bloc capsulectomies need to be seen for it to be added by your name under Explant Surgeons. You can attach them in comments or email them directly to [email protected].
ALLERGAN NATRELLE SALINE BREAST IMPLANTS RUPTURE, CAPSULAR CONTRACTURE, RIGHT IMPLANT LODGED IN SKULL, TUMORS SPINE, NECK, THROAT, DRAINING ENTIRE BODY INVOLVED, STILL ALIVE THROUGH GRACE OF GOD
Did both your implants rupture? I have the same exact implants 800cc only silicone. I have explant surgery on Dec 9th. How did you find this out through MRI? or CT Scan? I believe it gave me thyroid cancer. I removed my thyroid last year.
Hello! How does someone get in contact with the person in control of this website? My plastic surgeon is in Kirkland, WA and he specializes in helping women who would like their implants removed/replaced/etc. – en bloc resection of capsular contracture. He personally wants to help educate women who do not have the recourses to know the details of implants and what could happen if your body rejects the ‘foreign’ body.
Thank you!
Hi Stephanie,
This website can be reached by email, [email protected].
Stephanie – I had my last Silicone filled implants put in in the 1995 in Bellevue, WA. My last MRI showed that one of them for certain and the other one very probable – have split open and the Silicone has leaked into the scar tissue capsules. The surgeon who put them in is not longer working. My C-reactive protein number (that shows inflammation in the body) has been consistently between 10 and 11 (high enough so that they retest to see if there is something wrong with the test). I have had surgeries I could have probably avoided. My breasts now hurt and I really need to have these implants removed, along with the scar tissue capsule and free floating silicon in the capsule. I am now on Medicare with United Health Care as the secondary insurance. Could you give me the name of your doctor in Kirkland and I can call his office to see if they take Medicare? Thanks Stephanie. I am Carolyn . . . [email protected].
My explant/capsulectomy/testing is next Wednesday and I have just had my pre op appts. I Want to ensure my surgeon I ensure proper testing of all matter, as I have been VERY symptomatic :seeing oncologists/MRIs/RH docs/Lumpectomies etc etc Debilitating illness
Background: I was part of the Dow Corning class action settlement after I had severely bi-lateral swollen/ruptured/capsular contractured Textured implants partially removed (no capsulectomy/no testing of copius fluids that were collected for Bi-ALCL AND ruptured textured matter that was adhered to my body that still is there) 9 years later I implanted Mentor silicone implants and the surgeon noted several areas of the textured implants that he was ‘unable to remove’. After 10 yrs w these silicone implants I have have been severely symptomatic (burning chest cavity/swollen lymph nodes/debilitating fatigue/petechia rash/skin hot to the touch/confirmed silicone in lymph nodes/loss of appetite/night sweats/palor/low grade fever)
I know now that all matter/tissue/fluid can & should be tested, but I need the language to ensure that actually happens. At yesterday’s appts I was told he would ‘ask the lab to rule out BI-Aclc, but that is not sitting well with me.
Advice appreciated! Thank you
Hello,
I had silicone implants about 12 years ago. A little over a year and a half ago, I developed neuropathy (bruised and burning feeling) in my feet and lower legs. About two months ago, burning started in my hands and arms and the left side of my lips. I also have tightness in my chest and some thinking problems. I am so tired much of the time. My question is whether anyone knows weather BII can cause such conditions. I do not seem to have many of the symptoms I have seen on this site. I have been to 7 doctors and am scheduled to go to Mayo Clinic for more tests, but if these issues have resulted from the implants, I would like to focus on having them removed instead of spending more time and MONEY on tests. I am in so much pain and have even lost the ability to walk my precious dog. I am so sad to hear your stories on this site. I wish everyone healing.
Remove them asap. Refer to comment I just entered at 2:18 pm, March 19, 2021. Good Luck, Roe
Hi Christina
Did you have your implants removed? I had mine (silicone) implanted after cancer as reconstruction after a double mastectomy. It’s been almost 6 years and the past year has been havoc on me with increased neuropathy, tingling and pain with cold and hot sensations in my feet, heaviness in my lower legs, and terrible skin sensitivity in my entire legs. I’ve been through extensive nerve testing and numerous doctors to no avail. Not one of the doctors that I have consulted feel that my issues are caused from my implants but they are ok with labeling it idiopathic! I also suffer from visual issues that are very hard to explain. My eye doctors find nothing wrong with my eyes. My breasts feel fine and look great. I have no other pain but truly believe the implants are causing my issues. Just wondering What you did and if it helped. Thanks
One of my questions is regarding Dr. Shaher Khan. You include him in your list of Michigan surgeons, but I’m surprised he isn’t included in your Expert list and the other categories near the top of the page. Unless I’m mistaken, he has never, ever done breast augmentations. His entire focus (aside from hand surgery) is on Breast Implant Illness. He provides lots of educational videos and only does en bloc capsulectomies. I’m curious why he isn’t on the more elite lists. Perhaps you know something that I need to know before deciding on my surgeon. ? Thank you. (I hope you will reply to me by email soon.)
I had my enbloc March 10th and feel AMAZING. My vertigo, headaches, depression and bipolar symptoms are all gone. I can thank you guys enough.
I would like to speak to the website admin. I want to start bringing awareness to the Hispanic culture and would like to ask a question. Thanks
Hi Carniola,
Excellent on your recovery!
Thank you for your comment and email, a translate feature was added to the top right of the website to help with BII global awareness. Viewers can click on it and scroll down to Spanish.
What is the chance my silicone is going to explode? I have a uniboob and the surgeon placed the implants (800cc’s, the biggest I could get Medicaid to pay for) too close together. The silicone detached from my sternum and now I’m wondering if the two implants rub together, does that make them explode faster? The abrasion? What happens if the Coronavirus causes a silicone shortage and I can’t replace them? I’m so afraid of one implant deflating and the other one not. But maybe one implant exploding would help the uniboob situation…
Brigitte, March 12 2020 at 16:24
I had my saline implants removed 2 days ago with en bloc….also my surgeon Dr Parson from Scottsdale Arizona did a revision of my breasts with mastopexy. I am so happy I got these implants out…I feel calm inside, i did not have hot flashes in 2 days and my anxiety is almost to zero. I found this surgery minimally painful and I am relieved with tylenol. Dr Parson is very skilled and cares about your health. I am expecting to feel better day by day and be on the path of health and happiness…this brings tears to my eyes. I had my implants since 1998.
Hello Brigitte I was just wondering how you are feeling after removing your breast implants??? Did it help you feel better??? I’m desperate for answers!!! After reading all these comments I feel I found the issue to my problem.. My health hasent been the same after these implants…
I apologize in advance for the peculiarities of my translator. 3.5 years ago, I installed Mentor implants. After 20 days, I felt all the symptoms that other women often describe. I was very swollen, I had dryness, panic attacks, depression, chronic fatigue, hair loss. For the first 2 years, I considered it a consequence of physiological stress after surgery and a very difficult job. In the third year, I turned to endocrinologists doctors: subclinical hyperthyroidism( AIT), chronic adrenal fatigue (stage 3). 1.5 years I tried to treat different doctors and only one in the last 6 months has improved my health by 50% (but in these last 6 months I already knew about the disease of breast implants and was preparing for removal). In February 2020, I found a surgeon in Moscow who performed en block removal at my request and showed me photos. They gave me my implants-they were whole. He also performed a facelift (all of his work on the facelift is very good), but I was worried whether he could and would not refuse me a full explantation. In Russia we have many surgeons do not believe in the disease of breast implants. I am very grateful to the surgeon, endocrinologist, and your website (which reached Russia) for my second birth. Now I am recovering, but my chest muscles are hurting (but this pain is psychologically pleasant, since I know that there is nothing foreign in me). I wish all women a speedy recovery, as well as minimal difficulties in finding money for surgery!
Saline mentor implants. I was so sick and in so much pain. I had to pay out of pocket to get them out. I have photos of the calcified gross capsules. I’m posting everywhere to warn women not to get implants.
What were your symptoms??
How long did you have the implants?
To Whom It May Concern, We would like to participate on this site. Dr. Christian Drehsen is a Board Certified Surgeon that specializes in these explant procedures. Could you please guide me, in order to submit our information.
Thank you, Karina Brown
Hello ladies,
I am 3 weeks away from having these toxic shit bags out. I’m curious to know if anyone has ever dealt with foot pain or plantar fasciitis symptoms and if so did it go away after explant?
I had plantar fasciitis and started wearing oofos flip flops everyday and no more foot pain….
No connection. I have plantar fasciitis before and after explant. You have to treat the foot.
I have been going downhill for months, well since December of 2019. I was just chalking it up to pneumonia I got in February. I am normally quite active and healthy. I run an in home day care!. I struggle with heavy fatigue, especially with walking , etc.., and it’s worse when going outside. Night sweats every night! Getting headaches alot lately. I have dizziness episodes, where it feels like my brain is being zapped with an electrical current. Every joint in my body now hurts, and I am experiencing muscle weakness.
I am scared, truth be told, to get these things explanted, but am beginning to wonder what will continue happening to me if I do not get them out. I have Mentor, saline, smooth, round moderate implants since 2010.
i am dealing with that and many many other symptoms. I have my surgery in 2.5 weeks so i won’t know until then
I am a 32 year old mom fighting for her life for a second time, Please read my story and share if possible! I am trying to raise money for a surgery I desperately need but even more important maybe I can raise more awareness of this toxic illness, I wish I would have had the known then what I know now.
Read story on the gofundme link
gf.me/u/xfv6ia
Thank you in advance for sharing, God Bless
Skilled Enbloc Total Capsulectomy surgeon in Buffalo, NY. I explanted with success, November 2018 – I selected my surgeon because he is extremely skilled at this procedure doing at least 30 a year. Since my Explant I have lost 18lbs of inflammation and many of my autoimmune illnesses are improving and or like my wide spread Fibromyalgia have dissolved. I literally was on my last leg when I connected the dots and learned about BII and if it weren’t for this skilled surgeon I don’t know where I’d be today. . His name is Todd Koch, MD – 716 631-1220
– https://www.toddbkochmd.com/
Hi Alison,
Thank you for sharing your surgeon!
I have responded to three of your emails in regards to this (the latest one was today) and to your comment from January 21st under Resources (you may need to refresh the page to see it). I am not sure if you are receiving the emails.
Can you please submit a picture of his en bloc capsule/implant photos by either posting them as an attachment to a comment or emailing them to [email protected]? At least three en bloc pictures need to be seen for consideration of a surgeon to be added to the list.
Hi – I will get those for you. Thank you.
Hello:
My name is Jennifer & I work in Dr. Randy Rudderman’s office.
I wanted to supply you with resources from Dr. Rudderman’s website.
Dr Rudderman is currently on your list of resources/surgeons.
Note: Dr. Rudderman has opened a 2nd office in Midtown Atlanta
Dr. Rudderman has over 25years experience with explant surgery – he performs multiple explant surgeries every week.
Dr. Rudderman
1110 West Peachtree Street
#820
Atlanta, GA 30309
678-566-7200
https://www.drrudderman.com/before-after-gallery-atlanta/breast-implant-removal/
https://www.drrudderman.com/plastic-surgery-procedures-atlanta/breast/breast-implant-removal/
https://www.drrudderman.com/plastic-surgery-procedures-atlanta/breast/capsular-contracture/
I explanted with Dr. Rudderman April 2019. He and his staff, and the surgery center are wonderful! 💖
2016, Mastectomy R side w reconstruction. Allergan Biocell gummy bear. On the recall list. Plus im homeless on the street of San Francisco. I have really REALLY good Friends and support thru Shanti, an org that serves all women with cancer. I have had 2/3 of the symptoms. They started about 6mos after being implanted. I have learned a great deal about this but still have to find the right lawyer so i can sue Allergan as well as any medical professionals and healthcare providers i can. I will not hold back on that. It is the only way to effectively get the point across to the severely misguided people in the entire supply chain of these abominations. STAY STRONG FELLOW BREAST SISTERS. WE WILL SURVIVE!
I have had my breast implants since 1986.. ( months after having them put in I got sick and to this day still. Always tired, headache, have reflux and the list goes on and on. I went to the doctor and told her about all systom and told her I was thinking of having them removed. Being such a flat person when I had them put in, she advised me not to because my skin on my chest would be droopy. I just had a blood test done recently and it shows as if I am fighting off something in my body as my white cells are high and what nothing they can do. I wish I hadn’t done this but thinking I would fill better about my self. I can’t feel good about my self if I don’t feel good anymore.. Try to better your self and all your doing is making life worse.
omg! I have had mastectomy reconstruction w an Allergan Biocell gummy bear on the R side 4 years ago. Been sick every since, i have Pancreatic Exocrine Insufficiency plus about 2/3 of the symptoms on this website’s list. Be STRONG YOU GUYS!! IT DOESNT MATTER WHY YOU GOT IMPLANTS!!!!!!!!! YOU ARE INNOCENT!!!!!
The skin can be taken off surgically and re-connected so that the skin is tight. https://www.webmd.com/beauty/mastopexy-breast-lifting-procedures#1
Please believe me! We are all very very valuable creatures who deserve to be here and deserve consideration and kindness.
Keep reaching for support from your Breast Sisters. I’m right here with you! We here are All with each other always. Never forget that, especially when you are sitting there on the exam table with that gown on and you feel so powerless and weak. WE WILL SURVIVE:-)
Hi did you get them removed and not get replacements did your symptoms go away
Hello,
I had my silicone textured implants for 8 years. A year ago I began having weird symptoms such as uncontrollable cough, chocking sensation, asthma, acid reflux, spasms in throat, gastro problems, metalic and sour taste in my mouth, fatigue and vertigo.
I was in and out of the emergency room, doctors office and tests such as blood work MRI’s CT scans and everything would appear normal. Finally I saw a pulmonary doctor who send me to have a CT scan of my chest and that is where we discovered that both implants had ruptured.
I went to go see Dr. Zol Kryger in Thousand Oaks California and he squeezed me into his busy schedule to remove the implants that were literally killing me! He performed a full capsule removal through enbloc or total capsulecotmy.
My surgery was done in January 2019 and months later I feel back to normal.
I highly recommend Dr. Zol Kryger. His staff and his anesthesiologist were beyond amazing! They made me feel very comfortable and called me everyday to check up on me.
He is located at 947 Thousand Oaks Blvd. Thousand Oaks CA 91360 (805) 777-3877.
Feel free to contact me if you have any questions.
I’m a breast cancer survivor who had a bilateral mastectomy and breast reconstruction nine years ago in Texas. I had never been satisfied with the reconstruction as my implants were uncomfortable and I had felt ill and extremely fatigued since undergoing the reconstruction process. Over the years I have had many nagging health problems which I now attribute to the Allergan textured breast implants. After learning of many other women who have experienced the same symptoms and that this condition has a name: Breast Implant Illness, I started seriously searching for an explant surgeon. In the meantime, my textured Allergan implants were taken off the market, further motivating me to have explant surgery. Thankfully, I found this website and list of recommended explant surgeons in Arizona. I contacted Dr. John Rowley in Phoenix, Arizona who successfully performed the En Bloc/Total Capsulectomy explant procedure for me a few weeks ago. I was impressed with his bedside manner, understanding of my situation, and, of course, his surgical skills. He has an excellent team assisting him, as well. I am still healing but so relieved to be free of the implants and have received a clear pathology report. Some of my breast implant illness symptoms have already subsided and I’m beginning to feel like myself again. I highly recommend Dr. John Rowley!!!
Can u talk about the price
I got my first set of Silicone Implants in March of 2007 a year after my last child was born. I consulted with 4 surgeons before selecting a well known surgeon who had worked on a mega super star but was very conservative. I felt fine for the first 5 years, but then began feeling just a general overall sense of under the weather, tired all the time, and got a weird buzzing in my ear about 6 years into having them. I spend 2 years going to different medical doctors. I started having hormonal imbalances. My periods were no longer even close to normal and it appeared that I was starting menopause (early at at 42). I experienced ear “clicking” that was not tinnitis or a ringing but something that sounded like rhythmic tapping that seemed to correlate with some eye bouncing along with the sound that made me dizzy. I went to ear specialists, neurologists etc. No one really could nail it down. It was scary. One of my breasts seemed to sag much more than the other (under the muscle placement) so the 2 weeks before the 10 year mark (and out of warranty if there was a leak or rupture) I had them removed just in case there was a rupture or defect I wanted to get them out in the window as Ultrasound showed nothing but the appearance was marked. Again I did plenty of homework and sought out to find Breast reconstruction specialists and surgeons that focused on the breast. I found Dr. Pedro Soler. He was amazing. I replaced them with new tear drop, textured Mentor silicone implants. Within days to weeks I felt so much better. Within 4 months I felt amazing. A friend that was also in shape and active mentioned that she thought that her implants (saline and over the muscle but 15 years old) were making her sick. She was tired, but attributed that to having 6 kids and being a very active. She was having anxiety attacks, scalp sores, soreness, and was diagnosed with Lupus. I referred her to my surgeon Dr. Pedro Soler. To my surprise, he got her in to be explanted immediately. Within 2 weeks, she was recovering and decided not to replace with new ones. She was going to consider a fat transfer in 6 months or so when she was completely healed (although she does not have any fat to transfer and has a hard time gaining weight) It took her a few months to detox but she is no longer experiencing Lupus symptoms and is feeling overall much more healthy. I say all of that to give you back ground that there may be so many derivatives that play a part in why some women have symptoms and effects immediately and some no symptoms or bad health what so ever. I know there are studies that say both ways. What was amazing to me was that Dr Soler asked for both of us to come talk to him recently as he was researching the studies and latest information on BII and BIA – ALCL. He is a scientist first and foremost and wants to understand what the correlation is, read all the studies, read all of the boards out there of women who are saying they are getting sick. He wants to test the capsules, track his clients, figure out what the aspects and parameters that possibly play into the patients getting sick, be able to show and advise his patients, etc. I don’t know if my new implants will start to make me feel bad again or if the first ones were really the culprit but I do know to pay more attention to my body and I am so grateful and blessed to have a doctor that listens to his patients and does whatever they feel is needed to make them whole again. I chose to replace, my friend did not and we both feel so much better. He is hungry to find out what the real correlation is so he can prepare his clients and help the medical field be able to understand the association at the scientific level. I highly recommend him for anyone seeking explantation of their implants. Dr Soler has impeccable bedside manner, explains everything and is a great surgeon. He is in Tampa, FL. I will keep posting with updates as I get to yr #3 on these new ones. So happy there is this place for all the women (and men) to vent, voice their stories, seek answers and be a part of a family that understands your journey and is here to support and share. Thanks!
Hello…..I’ve spent over an hour reading all these stories about many & various symptoms and illnesses that most physicians can neither diagnose or find a treatment for. My daughter sent me this link, as she too seems to be suffering from symptoms like the ones mentioned in this discussion. She had had breast implants for nearly 23 years, and now has symptoms of gluten/celiac problems, migraines, and and dry eyes, , etc. Although I’ve never had breast implants, I did have to have surgery for a prolapsed vagina some 11 or so years following a complete hysterectomy in 1994. To repair the prolapse, the surgeon used a titanium mesh sling. Some years later, I began having diverticulitis, so much so, I had a Sigmoidectomy to remove the lower part of my colon. Then I had to have my gall bladder out. After that, the chronic diarrhea, and now 20 years later suffer from gluten sensitivity, to the point I experience chronic diarrhea which just infects the fecal bacteria up into the urethra and causing chronic, recurring UTI’s – It seems round after round of Rx’s for antibiotics, which further kills my immune system in the gut, causing more diarrhea, and round & round. I’m beginning to wonder if the Titanium mesh sling implant could be the culprit here, after reading all these postings. I hope to hear someone’s response to this supposition
If you suffer from ongoing UTI, please do not take antibiotic CIPRO do not under any circumstance, my daughter has for 2 years from a dramatic CIPRO reaction and debilitating illness, affecting many systems also addressed in Breast Implant Illness.
Wish you luck, and some healthy prospect
Since your gall bladder has been removed, you’re supposed to be taking bike salts with every mean, or you’ll probably have diarrhea.
Removing gluten and dairy from your diet will greatly improve your gut issues.
Omg my Mom had a hysterectomy and Vaginal mesh and her symptoms almost mirror yours! She has had diverticulitis, her gall bladder out and now chronic diarrhea. I suffer from BII but I never thought about the mesh causing her body to have similar symptoms because of the mesh. But the problem is, unlike implants they can’t be taken out without negatively impacting your internal organs, can they??
Thank you so much for this very informative website which has answered so many of my questions. The explanations of how implants damage the body make so much sense to me. I am a man who was fitted with a testicular prosthesis (basically the same silicone implant as a breast implant) almost twenty years ago following an accident. I was already suffering from ulcerative colitis which was well controlled by fairly mild drugs. A few years after the insertion of the prosthesis I suffered major flare-ups of the colitis which badly affected my career and social life and I’m now on strong immuno-suppressant drugs and suffer badly from fatigue and depression. I’m scheduled for an explant in a few days time and I am now hopeful that my health will recover.
Hi Ray,
Welcome! Glad to have you here and to see you are preparing to explant your silicone testicular prosthesis. Thank you for sharing your story.
For all viewers I would like to point out that regardless of gender, the common denominator is a similar pattern of symptoms following implantation of silicone foreign bodies. There are other males that have reached out, below there is a David who had a silicone chin implant and on the Symptoms page Charlie and Jeremy both have commented on having testicular implants and health issues.
If you would like to contact someone with a similar experience, Jeremy had testicular prosthetic explant surgery and left in his comment “Feel free to email me if you like. I know what you are going through and it sucks. My gmail [email protected].”
Dear Admin,
Thanks for your reply and for giving me those contact details.
I had my testicular prosthesis explant three days ago and all has gone well. I am already feeling much better simply knowing that the implant is gone. Thanks to the information I have learnt from this wonderful website I was able to explain to the surgeon that I wanted that capsule removed as well as the prosthesis itself. I’ll let Mother Nature heal the wound and I am very hopeful that my overall health will improve. Life is a journey and we learn so much along the way.
This website is a fantastic resource. Good luck to you all!
Can BII cause low sex drive? My mother referred me to this website because she belives my low sex drive could be linked to the implants I got a little over a year go. I’m in my early 20’s and in a great long term relationship. I got a hormonal IUD about 8 months ago due to negative side effects from the pill. I originally thought my IUD caused this issue but when I started telling her about some of my other symptoms like increased food sensitivity, brain fogginess, memory loss, etc she sent me here. I don’t see anyone mentioning low libido so I thought I’d ask. Thanks for the help!
I forgot to mention fatigue! My point is that someone of my age and with my health shouldn’t be experiencing these types of symptoms so frequently. Any advice helps!
Brooke
I’ve been battling this for two years now
And same thing ! They thought it was my IUD AND and now two years later (I did convert to
The nuva ring ) I’m at a stand still. I have zero energy have been in and out of specialists with no answers just “generalized auto
Immune” make sure u ask your Gyno
About endometriosis can do a lot of the similar effect BII DOES..
I’m looking into removing mine:( I’ve only had them two years
Hi Brooke,
Silicone is an endocrine disruptor and many experience decreased sex drives that tend to resolve after explant. IUD’s can be made out of silicone or copper and also cause problems. Women have complained of silicone poisoning from the Mirena IUD as well as other health issues and there are many lawsuits.
After 26 years of having silicone implants in my body, I had them removed this past April. The nurse brought them to me after surgery so I could inspect them. They were not broken down, looked new, the saline inside was crystal clear, and didn’t have any odor. My question was answered of “how can implants break down in the body when it is said to take 1000 years to a plastic grocery bag to break down in the landfill?” Well, it doesn’t take the implants to break down, or fungus growing inside the implant to make one ill. It’s just having the foreign object in the body that is causing the immune system dysfunction. I feel much better now, and know they were the cause of my immune system problems.
hi Marie what doctor did you go to and are you happy with your breath shape and was the doctor able to reconstruct the breath back to normal
sorry for the misspelt word that meant to say breast
Any advice on what to do to help manage symptoms until I’m able to have them explanted? I have a constant itch and rash that will not go away.
Blood work, pathology all came back perfect. Steroid shots, creams, nothing helps but I can’t get surgery for at least a year.
JY, I sympathize with similar skin problems, It’s amazing how miserable a rash can make you feel. Try adding antihistimine to your regime. Worth a try. Topical cream and/or oral medication. Good luck.
Wondering if there are any other males with lots of strange symptoms. I was born with Poland’s Syndrome, which had me born without a right pec muscle. When I was 31, I had what the plastic surgeon called a “semi solid silicone” implant implanted. I’ve had so many bizzaro symptoms and they’re getting worse. Panic attacks, numbness in limbs, acne and rashes, and in the last 10 month, I started getting alopecia bald patches with the hair growing back in white slowly after 6 months. I aged dramatically, I feel, in the last 2 years. I also have the worst brain fog and short term memory loss. People tell me I promised to do something a minute ago and get mad at me and confront me when I don’t so it—and I have no recollection that the convo took place!
So, I had chose the softest firmness level of the options and only one implant was implanted. It is silicone; however, it is “semi solid” and without liquid—it’s like a very firm Jello jiggle sculpted to mirror my left pec and was inserted under my armpit hair so as not to leave a scar. My doctor said before I the operation, that the silicone can’t produce any symptoms; he said that only the leaking gel breast implants for women caused problems. It’s been about 10 years now. It only occurred to me recently that maybe my autoimmune response with the alopecia was like the autoimmune response a woman was having with her implants. How horrible. I don’t think I’ll have the time or money to have this removed until a year from now.
Stephen, so sorry you are not feeling well. Your doctor misinformed you. Implants do not have to be leaking or ruptured to cause symptoms. silicone implants break down in your system even if they aren’t ruptured. Do what you need to do to get it out asap. 10 years is way past the mark of keeping it in your body.
Hi there and thank you for this very informative website. My name is Kim and II have suffered with breast implant illness. I had surgery two days ago and was supposed to have an en-bloc total capsulectomy. I found out later that day that the surgeon left the capsules in. I am devistated and am now wondering if there is further toxicity now because of disturbing the capsule. He obviously had to cut the capsule open to get the implants out. Also, now I need a second surgery, and wondering if it will now be more difficult to get out the capsule.
Is there anyway to find out why Dr. Pousti of San Diego is no longer on the recommended page of surgeons? He used to be on the list and I have a consultation appointment with him for explant but first I wanted to be sure there wasn’t a reason he was removed.
Hi Maria,
Dr. Pousti does not always remove the capsules. In March 2018, he wrote on Real Self:
“In my practice, generally speaking, unless the breast implant capsules have thickened and/or are otherwise symptomatic, I do NOT remove them.”
Full capsule removal correlates with recovery. Some women in the BII community have remained symptomatic and needed second surgeries to remove capsules left inside by surgeons. After capsule removal they had their health improve. In all cases they should be fully removed upon explant.
Attachment BII-SEMINAR-UPDATE-5-28-19.pdf
Good Morning,
Our practice is devoted to BII. Dr. Shaher Khan refuses to put in implants. He is a Board Certified Plastic Surgeon and also a Board Certified General Surgeon. Dr. Khan truly belies in BII and would love to help all women in need. How can we be recognized on this website like all the other physicians. WHo can we talk to? I have attached a flyer for our BII Information Seminar coming up on 6/18/19 and we will streaming facebook live as well. Please contact me to discuss further. Lori Welch- Practice Manager 734-620-3767, you may also contact Dr. Shaher Khan direct as he would love to speak with you himself 914-316-8055.
Hello, my names Ashley. I’m 25. I had implants put in seven months ago. Have had weakened immunity ever since and these are some of my symptoms:
Insomnia
Burning/chronic chest pains
Nonstop Mouth fungus
Recurring tonsil stones
Swollen lymph nodes and jaw
Migraines
Chemical sensitivities
Eye swelling
Recurrent sinus/allergy infections affecting face, eyes, ears, and throat
Chemical Metallic taste in back of mouth
Mood swings/raised anxiety/depression
Feeling like I’m dying
Heart palpitations (scary)
Weakened immune system
Extreme fatigue
Unexplained weight gain and extreme bloating despite healthy eating and exercise
(Body inflammation)
I’m thankful to the women putting information out there to help others fighting for justice and to get their life back via explant. So sad to think my health was perfect right up until I had these implants put in. I am having surgery next month to explant. DO NOT GET IMPLANTS PLEASE to anyone considering it’s not worth the agony they cause mentally, physically, emotionally, and financially speaking. Our bodies are perfect the way they were created. Don’t ever let anyone convince you otherwise.
Best wishes to everyone out there suffering like I am….
Hi Ashley,
Your story is our story. Thank you so much for sharing. If you haven’t already, please submit a FDA MedWatch Report. Additionally, there was a FDA public advisory meeting on March 25-26 2019, and women may submit comments on breast implant experiences to the open docket until April 26th.
Hi, I’ve had my implants since December 1990. I live on the coast of Mississippi. Can you recommend a surgeon in the area? Alabama, Mississippi, Louisiana?
Ladies, I’m thankful when reading your thoughts and health issues might have a relation with breast implant. Today, I happen to see TV news mentioned on this subject, same day, a friend forwarded to me this article. By knowing there’s a chance to become healthier, helps me to speak up about my issues without feeling ashamed. I’m 68 yrs young, came to the US at age 23. I have a total hysterectomy (due to the awful endometriosis) in 1988. My first implant with silicone was 1975, from flat chest to a reasonable figure. I was happy with my decision and regain my confidence. I have mammogram done every year, the results always “no issues”, and I don’t feel any pain at the area in all those years. The 1st replacement of the implant, and the 2nd replacement (due to infection), both were done in 2013. The reason I was thinking of having the replacement, because I saw an article saying implants shouldn’t keep in the body more than 10 years (another article saying 5 years). During the work of the replacement, the doctor told me that my old implants had become pieces with many capsules. I don’t recall since when, I have been dealing with more and more health problems. I have 15+ physicians / specialists tried to help with my illnesses, and all the comments returned that my problems were caused by stress. Also, the lab-works, reports, always saying “no negative issues they can find”. The physician will either recommend for a different RX intake, or add on more RX or other procedures, even surgeries, in the past years. Other than all the health problems had become worse as time goes by, my GI area… the current major issue, at this time… had become unbearable.
These are the symptoms related to illnesses that I have to deal with daily, listed below:
Depression; mood swing (bipolar); MDD/GAD/ADD; social phobia; anxiety; panicking; chest plan; suicidal thoughts (thinking what’s the point to live with all these pains); IBS (ulcerative colitis) created more runs and also created a very bad hemorrhoid issue (no avail to get better after 3 procedures); abdomen pressure/bloating; sleep apnea; dry eyes; year round allergies; lower back pain; arthritis; joint pain; numbness hands; heat intolerance; vertigo; dizziness spell (this cause an accident with 5 cars in front of me stop at red light); generalized weakness/fatigue; weaken/low immune system; migraine headache; sinus headache; loss memory; loss hair; and losing my mind. The new comer is splitting; brittle; soft and thin nails. I’m sure there’re some illnesses being forgotten to add to the list
The mentioned symptoms either major or severe, most of them are currently on daily RX medication + over counter’s, but still getting worse on a daily basis. I understand some of the symptoms getting worse because of aging. Therefore, I can’t say they’re all related to the implant.
After reading most of the information, I think my hope to live is to get the breast implants remove. I’m not sure where to start, because I don’t have a surgeon that I can fully trust for this procedure. I have trust issues with the world of medicine for years. I have been working very hard to get a better health so I can say “happy to be a life”, as I’m still alive.
That’s all for now, because I’m getting very depressed by putting my problems in writing.
Good outcome I am 2 months post explant surgery. My implants were in for 30 years. I was told they were saline. When any issues presented during this time I attributed it to allergies and so did my docs. The entire time allergists thought I was extremely sensitive to a lot of things and treated the symptoms. During the last couple years strange symptoms and yes all of the classic BII autoimmune syndrome sent me to 8 specialists with the same outcome and yes I did start to suspect that even though they were saline that they could be the culprit. In general I felt like I was shriveling up and drying out. I felt like my immune system was fighting something to which it was always successful at but this time it was fighting a loosing battle. My body was telling me something and I finally listened. Visited a surgeon that I knew and trusted and he boked my surgery in 2 weeks. I appreciated him taking care of me quickly because I was frequenting the emergency rooms with what they tagged PANIC ATTACKS the doctors were puzzled at why they could not control my BP or heart rate which was causing me aching and mimicking a heart attack. All tests done by all specialists including for rheumatoid or autoimmune were all negative. My recovery is amazing to me I feel 20 years younger and mentally feel refreshed. My ringing is not as bad but remains. I have recruited some help with supplementation to facilitate my recovery of any soft tissue that is stressed from local vitamin stores. Hydrate is my mission. If you have any of or all the symptoms don’t hesitate to invest in explant surgery ASAP . They can sabotage your lifestyle in more than physical symptoms it also can strain your relationshps with friends and family. Breast implants will always be a part of our society but I just believe that proper maintenance should be considered before you commit to them. The worst part is we are so used to our physicians catching something like this. Plastic surgery is an elective surgery and financially we are on our own with it including maintenance. They should have physicians give an overview of maintenance plan to catch any complications.
Lorraine – your story is so like mine. I’ve had my implants since 1983 and just learned they were never meant to be permanent. I also have 20+ symptoms of BII for which I am being treated by several different physicians. None of my doctors will say it has anything to do with the implants. I am now seriously considering removal but wonder will having them this length of time and then removal leave me deformed. Without being too personal, did you find this to be a problem? I am 65 yrs old so I know some illness/issues are age related, but stroke level BP,IBS, anxiety/panic attacks, RA, OA, and fibromyalgia, eye infections, asthma issues; heat intolerance among them seem to be getting worse daily. I have had to have my thyroid and gallbladder removed because of illness and neither of these have relived any symptoms. Your thoughts on this would be appreciated since you are the only one that I read about who has had the implants nearly as long as I. Thank you for sharing
Colleen, I have many similar issues as well. Since my post didn’t receive any reply, do you get some advice that isn’t shown here? I wasn’t able to locate for a doctor for explant surgery, and hoping someone with experience will have an idea where to start. I’m getting more worried every day!
Hi Donna,
Your story is our story; from your long list of symptoms to the lab work coming back with “no negative issues they can find” to the 15+ physicians and specialists. The path to healing begins with removing the breast implants and their surrounding capsules. Start by reading on proper explant and understanding the importance of full capsule removal. Next, see the list of explant surgeons and if interested you can join Facebook BII support groups where you can ask other women questions on surgeons used and see pictures. See the list of questions to ask explant surgeons and set up surgeon consults until you find one you are comfortable with and who is experienced in full capsule removal.
Hi Everyone,
Really hoping I can get some insight/help here since I am feeling so lost. I’ve been dealing with some issues myself, but the one thing that is REALLY taking a hit on me is my anxiety/panic attacks. I’ve never had a history of either of them; however seems to be getting worse and worse to the point where its scaring me. I am literally worried about my mental health. I constantly have to talk myself down and have woken up in the morning already panicking that will sometimes last throughout the day and just continue in waves.
A little back story on me: I had my first BA in Jan 2013- everything went smoothly and I was fine. I ended up having two kids, still feeling fine. I started to experience back/neck pain but thought it was just due to having/dealing with my kiddos. It wasn’t until 2017 where I started to experience anxiety and panic attacks (which I didn’t know I was having at the time). With that, along came crazy headaches, feeling dizzy, sick, lightheaded and my right eye would always be swollen and never knew why. I finally went to the doc – they did every test possible but everything came back fine. My headaches wouldn’t stop so finally the doctor sent me to get an MRI in Nov of 2017 – they found something small on my left frontal lobe but said that my symptoms had nothing to do with what they found. Of course this sent me into panic mode, esp. being a mother of two young kids and not knowing what is going on with you. I went to three different neurologists, they all said they had no idea what it was – that it could be anything from a fall I took as a child that left some scar tissue to a small tumor. Anyway, we’ve kept an eye on it and it hasn’t changed in size but still dealing with everything else. The beginning of 2018 things started to get a little better (physically and mentally) for whatever reason…maybe because I started to workout and keep my mind busy. So, I thought it was time to get my breasts redone….(yes, I know….this was before I knew anything about BII). So, in May of 2018 I went bigger and was feeling pretty good. When my surgeon took out my old implants, one looked a little deflated which I thought was weird but he didn’t seem concerned and said everything went smoothly so, I didn’t give it too much thought. I recovered and shortly after (in Oct of 2018) I got this really weird rash that wouldn’t go away. I went to the Dermatologist and he told me it was either Eczema or Hives. I couldn’t believe that since I never had a history of skin issues… It lasted a month and then just randomly went away. The following months Nov/Dec I started to experience numbness in my hands and feet, heart palpitations, and my anxiety/panic attacks where at an all time high to the point where I told my s/o that I thought I needed to go to the hospital because I couldn’t calm myself down. I didn’t want to be too far from the house, didn’t want to go to dinners or movies anymore. I was just so worked up with no reason to be worked up. I can’t pinpoint where it comes from besides just worrying about my mental health. Ever since then, I’ve been dealing with the anxiety more than ever. My question is, everything else tends to come and go, but my anxiety seems to always be there. I have no idea if this anxiety has anything to do with BII or if its just me and I need to get on medication. I just wanted to reach out and ask if this is something that hits home for for anyone out there…If so, I would love to hear your story. Thank you for your time.
Hi Nicolette,
Anxiety and panic attacks are very real symptoms of BII. I developed them with breast implants and so have thousands of other women. I went into a state of isolation and gradually deteriorated into a shell of the person I once was. Rashes, heart palpitations, and numbness in hands and feet are other symptoms of BII. Mental and physical health return with explant. There is hope, the heal is real.
So I have recently noticed that I have a brain fog I don’t feel like myself and just my mental state has gone to crap!!! It’s very scary and I have never felt like this in my life!! I as well am experiencing a lot of the other symptoms but the one that bothers me the most is my mental state I have only had my implants for a little over a year and my life has been flipped upside down I finally got the nerve up to say something to my s/o and I don’t feel as crazy talking about it after reading all these post !! I have also found myself isolating and having social anxiety which I never have had !!! Im seriously thinking of having them explanted and idc if my boobs are ugly afterwards I just want to feel normal again
As with so many other people on this site, I can’t tell you how amazed I am that soooo many people are experiencing the same side effects. I am a BR CA survivor and had a double mastectomy in 2016. I then had chemo/radiation. Shortly after, I began feeling joint pain, sharp shooting pains in my joints and neck. A couple of months later, my jaw locked and hasn’t re-opened to full capacity. I’ve had several surgeries to correct the mis-alignment of the implants and even had a surgery to try and open my jaw – none of which have worked. I began having hot flashes and severe depression which I am now taking several medications for. I have been to numerous providers who said it’s probably just the side effects of chemo. I am tired of feeling tired! I have a consultation in June to have these implants removed.
My question is – is there anyone on this site who has gone thru chemo and then had the explant procedure? If so, what were your symptoms prior to the explant procedure? Do you notice a difference now that you don’t have implants?
I had stage 4 breast cancer and had chemo and radiation. I tried to have my breast implants removed due to pain in but my doctor bullied me into getting smaller ones instead of removing them completely. Then I had an acute infection occur a month and a half after they put the new implants in and they were forced to perform an emergency removal of the newer implants. I have had all the symptoms of breast implant illness – even after their removal. I am still trying to get my records and find out if they did a total or sub-total capsulectomy. I must be in the 20% that did not improve post implant removal.
Is there a doctor that believes in silicone disease in the Dallas Fort Worth area? I need one as soon as possible.
I have heard great reports for Dr. Melmed in Dallas. I’m not sure why he is not on this list of surgeons. He has some You Tube videos. Hope this helps!
Hello ladies! Anyone who had ear ringing with implants , after Explant did the symptom disappear?
I would like to know the same thing : ) I had saline implants about 20 years ago and that is about the time when I noticed ringing in my ears. Over the years my Tinnitus/constant ringing in the ears, has become worse to almost debilitating levels. I never connected the two … now I wonder. I would literally give up my right arm to get rid of the sound in my head, maybe I just need to give up the implants. hmmmmm, I’d appreciate any insight. Thank you
I want to know the same thing. I’ve had my implants 21 years and 6 weeks after I got mine I was suddenly slurring couldn’t drive or walked in to walls symptoms subsided and then came back but I was diagnosed with MS so I didn’t think it was implants. My ears started ringing in 2012 until now I’m 2019. SO LOUD. I’m saving money for explant and wanted to know if I can hope for them to stop upon removal.
M.S. is linked to BII. Also BII is linked to demyelination that doesn’t fit any specific pattern of demyelinating disease like M.S. I’m so deeply sorry…the good news is I’ve read on other websites that it’s possible to stop & even reverse the demyelination by explanting, which as far as I know isn’t possible to stop M.S. let alone reverse the myelin damage with medication or any other treatments, yet when the implant/s are explanted, the M.S. is “cured”
Me too. I have been suffering with this ringing in my ears as well.
Someone please respond in regard to ear ringing. I am dying to know if this can be cured after removal.
I had breast cancer in 2015 with a double mastectomy (early stage so no chemo or radiation). I got my implants and pretty quick after that my hair started to rapidly fall out and I have a constant feeling of being hung over. I feel “odd or off” every single day of my life. and then the ear ringing started and it is getting louder and I am experiencing hearing loss as well. Maybe if the ringing would stop, I could actually hear.
I asked my plastic surgeon and the breast surgeon if they ever had someones hair fall out after implants and they said no, it must be stress. I have almost every single symptom of a bad thyroid, but my thyroid is just fine.
My Dr just wants to use an antidepressant to help me but that is NOT THE PROBLEM. something is really wrong in my body and I feel it every single day.
PS, I live a holistic, clean lifestyle with chiropractors, supplements, CBD oil, plant based diet, no chems, no sugar, no gluten, no dye, no meat etc and all of the work I do is only a bandaid. Nothing has cured me.
and PSS, all of my tests say I am “FINE” and I am NOT FINE.
So please someone respond so I know there is hope.
thanks!!
Hi Monica,
Ringing in the ears (tinnitus) is a symptom of BII. It is listed in Allergan’s Clinical Study (see subsection 3.7 What are other clinical findings? Page 30):
In Allergan’s Core Study, numerous signs and symptoms were collected at 2, 4, and 6 years post-implant. For primary augmentation patients at 6 years after implantation, statistically significant increases were found for the symptom category of Joint (includes joint pain, stiff in morning, swelling in other joints, and swelling of hands), Muscular (includes back pain, muscle pain, aches, or cramps, muscle weakness, neck pain, paralysis of arms or legs), Gastrointestinal (includes constipation, diarrhea, gastrointestinal pain, heartburn, loss of appetite, stomach pain or cramps, and vomiting), Neurological (includes headaches, loss of balance, numbness/tingle of arms or legs, problems with memory, problems with thinking, and ringing in ears), Urinary (includes problems with urination and urinating too often).and Fibromyalgia (includes back pain, fatigue, neck pain, pain, and pain in the chest).
In Dr. Arthur Brawer’s research, Chronology of systemic disease development in 300 symptomatic recipients of silicone gel-filled breast implants, 20% of of women developed tinnitus. In the Facebook breast implant illness community, many women have reported it going away with explant. It may not go away for everyone though as some do not fully recover from all the symptoms of BII. This can depend on many factors, one being how long one has had breast implants and progression of symptoms.
The article ‘I Wouldn’t Have Gotten Breast Implants If I’d Known This Would Happen‘ sounds similar to your story. We all have the commonality of sharing similar if not the same stories. She states:
“I went to doctors, of course, but all of my tests came back normal. … I ate organic food, did yoga, meditated, took care of myself, yes. Was it possible that I had the worst toxic soup planted right on top of my most crucial organs? … After doing my research, I knew what I had to do.
I had my explant surgery in March of 2018. A day after the surgery, two of my most persistent symptoms disappeared. A week after surgery, I started feeling more and more myself again; my eyes cleared, and my skin started to return to its normal color. Today, I’m four months post-surgery and celebrating the fact that most of this seems to be behind me.”
In another story, Breast Implant Illness? Sharing Ashley’s story, a woman had ringing in her ears as a symptom and it cleared within 6 months of explant. She also lists a good detailed detoxification and diet she did post-explant to help her body heal from the breast implant toxicity.
Hi Monica who was your doctor who did the explant. My name is Christine
I am using some of this data for a document I am writing on Idolatry of Beauty. Who do I cite when using your data?
Hi Adrianna,
Please email me at [email protected]. Thank you!
I’m so happy I found this site, because I was beginning to think I might be crazy. I’ve had silicone implants for two and a half years and I was hospitalized in October of 2018 for extreme fatigue, numbness down the entire right side of my body and sudden weight loss. The MRI showed brain lesions consistent with Multiple Sclerosis. It was 3 days of massive doses of Prednisone that finally pulled me out of it. Long story short, the MS tests all came back negative and the doctors have no idea what could have caused the MS-like symptoms. They were even beginning to think that it was a rare autoimmune called Devic’s Disease, only to have that test come back negative as well.
I’m getting the things out! But my question to you all is this – I just found out I’m pregnant, due in September. This is my first baby, and I want to breastfeed, with all of my heart! But I’m terrified there is some level of toxicity I could pass to my baby and of course that is the last thing I want to do. Please, any advice that you have would be so very much appreciated. If only I could get them removed before the baby arrives, but as far as I know that’s not a possibility.
Thank you so much.
I had symptoms similar to yours and very much like MS…they diagnosed it as Functional Neurological Disorder. It may be worthwhile looking into.
My wife just finally figured it out after almost twenty years of sickness and even had a hysterectomy and had all her teeth removed she had a bilateral mastectomy as a threat of cancer and they gave her the implants for free because they were supposedly doing a test or she immediately got sick with all the breast implant illness has dear God I wish we could figure this out before she’s just had a horrible horrible life but look you definitely have breast implant illness you need to get those suckers taken out no matter what she’s on it in a group on Facebook for breast implant illness and I don’t really know who they are but if you can text me back here or whatever I don’t know how this works we’ll try to get you in contact me with the sites that she’s found I’m going to give you this one here we found this pretty good https://www.youtube.com/watch?v=hdrnkhiNF6M&t=4s
Breast Implant Illness is so real!! – I’m 8 weeks post op from having my implants removed today and have no regrets! I feel so much better!
You can read about my journey here: https://www.joyfuljes.com/post/breast-implant-illness
I’m have explant surgery/mastopexy next week. I wasn’t offered the option of simultaneous fat transfer. Is it best to do both procedures at the same time? Is it better to do the explant/lift and wait to do the fat transfer? Help!
Hi I have a family relative who has breast implants and suffers from Sarcoidosis which is an autoimmune disease. Many of the Sarcoidosis symptoms are the same as the symptoms associated with Breast Implant Illness. Recently she’s been experiencing vertigo and silent migraines along with all the other symptoms associated with her illness. Has anyone on this page experienced the same? She has had many tests, MRI’s etc.with the results being as expected with someone who has Sarcoidosis. As far as I know not one doctor has suggested her illness may be a result of breast implants. To say the least I am very concerned. Thank you.
Hi Lois,
I got diagnosis with sarcoidosis of the Lungs An skin. I have my implants in for fourteen years . An I’ve been going through hell for nine of them. I can’t tell you how upset I am
For putting implants in. I put them in An didn’t realize until maybe four years in I started having some health issues but I didn’t connect to my implants. I will getting them out
She should get them out. I have all of the bii symptoms.
Hello Lois, I was just told I have sarcoidosis by my lungs, and I am now reaching my 16 year old saline breast implants. Did you have your implants removed?
I have been following this page for a little over a year now after experiencing my own complications from breast implants. Please take a moment to read my abridged story at the link below. Thank you!
https://www.gofundme.com/life-changing-tmj-jaw-surgery&rcid=r01-154763770873-72067f691e6e409d&pc=ot_co_campmgmt_w
I was diagnosed w scleroderma in October 2018. Been suffering for year of symptoms and IBS issues. Most symptoms I thought were my Epstein Barr virus. So I just pushed forward everyday. I’m new to learning about scleroderma and stumbled across the information on breast implants. Just found all this out tonight. I’m appalled. I never knew. I was never warned of all of this. I only have 10% useage of my hand and arm now. My implants obviously need to come out. I’ve been on ST disability from work. I have no money due to my medical issues. I may never return to work. What am I supposed to do? Insurance won’t cover a removal, correct?
Tell your story and start a go fund me. My Mom had Scleroderma due to implants she had as a result of double mastectomies. I also had double mastectomies and chose to have implants, i worry every day that I will suffer like she did.
Hi Bonnie,
Scleroderma is a common autoimmune diagnosis with breast implants, the two were linked in the 90’s Dow class action and in recent studies. In September 2018, the FDA released US FDA Breast Implant Postapproval Studies, the largest and longest study ever published of breast implant outcomes. Results indicated silicone breast implants are associated with higher rates of autoimmune disorders such as Sjögren’s syndrome, scleroderma, rheumatoid arthritis, stillbirths and melanoma cancers. Silicone is an adjuvant (immunostimulant), it provokes an immune response and eventually creates immune dysfunction and autoimmune responses.
Insurance does at times cover breast implant removal, the process of getting approved is generally not easy and requires persistence. Obtain a copy of your insurance policy and review it to see if they are likely to pay for the removal.
Dr. Diana Zuckerman has lobbied for insurance coverage of explants and has previously commented: “Many women are able to get Medicare, Medicaid, or health insurance companies to pay for the removal of breast implants if removal is “medically necessary.” Medically necessary can be leaking silicone gel, chronic pain from capsular contracture or other reasons, infections, ALCL, and some other reasons. The National Center for Health Research is offering free help for women who want to try to get insurance or Medicare or Medicaid to pay for removal. Go to http://www.breastimplantinfo.org and learn more about this opportunity, or take the very short survey linked to that page to ask for our help.”
I am an ER nurse & was hospitalized this past summer for asthma exacerbation, unresponsive to treatments. After 4 days of being in the hospital with no improvement the respiratory therapist & I suggested we infuse magnesium sulfate, which is a drug we use in the ER when everything has failed & we’re considering intubation (putting a patient on a ventilator). It finally worked after a few hours & I was sent home 2 days later. One month prior to that I was rushed to the ER in respiratory distress from my primary doctor’s office & diagnosed with allergy induced asthma. I quit smoking 6 years prior to that, so all of my doctors (which were also my co-workers) were stumped. Allergy testing post hospitalization revealed I was allergic to dust mites, but the wheals on my skin weren’t big enough to suggest a severe allergy to dust mites. 3 months ago, I became extremely & permanently fatigued, it seems. I lost 40% thickness of my hair because it was falling out in clumps. I felt like a cancer patient every time I brushed my hair. I have ADHD, with symptoms increasing exponentially including brain fog. My energy and stamina are for crap. I work 12 hour shifts in the ER & need 2 days to recouperate. My seasonal allergies are year round, 24/7. The endocrinologist said I showed signs of thyroid inflammation 2 months ago, but now all of my tests are normal. She can’t figure out why my symptoms come on vigorously then disappear a few months later. ANA tests & RA tests are normal. Working full time and going back to school full time, on top of these symptoms has me feeling like I’m going to lose my ever loving mind. I have no libido, and my poor husband is suffering because of that. I get moody & tired so easily. I’m sick with sinus infections and bronchitis on the regular. I’ve been to physical therapy twice in the past year for upper back pain which never seems to go away completely. My urine has a funky odor, but no signs of infection when tested.
Ihad an article on BII come across a news feed spontaneously one day a few months ago, as if the Universe was sending me a message. It’s ‘2019 & I have had my saline-filled implants since ‘2001. But, like a stubborn a$$, I ignored the article initially. Again, the news feed popped up this past weekend & I read the whole article on BII. I finally feel validated! I finally feel like there’s a light at the end of the tunnel. I am contacting the plastic surgeon on Monday to get these suckers removed. I’m so pissed that I didn’t figure this out sooner. My husband said if insurance doesn’t pay for it, we will max out our credit cards to get it done. He wants his wife back! Also, for about a year, my massage therapist has been telling me that she feels tiny little beads of some type of substance all over my back muscles every time she gives me a massage. I feel them too in some of the places I can reach, but the doctors don’t have any clue. In the CT scans and MRI I’ve had done in the past year, there has been no mention of these at all. I will come back here after a few months from explanatation & let you know if my symptoms have improved. I hope my story can help others not feel so lost and alone.
Holy SHIRT Katie! Me too, me too, me too. I heard the tail end of an interview using the term Breast Implant Illness yesterday morning, and googled it last night. After reading just a page or two I then spent the night coming to terms with my own stubborn, vain a$$. Whether or not insurance covers the explant is irrelevant to me, I have none. I lost insurance coverage due to divorce, then I was denied acceptance for new policies. If there was any insurance who would take me on, it was outrageously expensive and don’t forget the preexisting conditions issue. I don’t have the luxury of group insurance thru work because I lost my career. Then After building my own business, I had to shut it down due to extreme BII symptoms shutting me down.
I am grateful to you and all the others. It’s time to help someone else as I too put my health & life back together. it won’t be easy but it will be worth it!
Katie,
Your story is my story.. I too was an ER RN. Been ill for years with the strangest things. Now I have enlarged lymph nodes throughout my thoracic cavity. No diagnosis can be found after many, many CT scans, MRIs, PET scans, blood work up the ying yang. Needed bones in both hands removed due to destruction. Collagenous colitis, hair loss, migraines that only resolve with Toradol 60mg IM, depression, and the list goes on. Planning Explantation in Nov 2019. Last surgeon in 2015 put in textured implants, moderate high profile, 350ml. I SPECIFICALLY told him several times I want smooth…regular implants 325ml. WTF? I didn’t know this until I saw a report about textured implants on the news. I pulled out the ID card of my implants and was horrified that I hadn’t read it sooner. I’ve had both silicone and saline implants, 7 sets because they all ruptured. How the hell did I not realize what was happening to me? All my surgeons said there was no danger and I would look destroyed if I didn’t replace them. I’m 65 now and have had implants since I was 22. I don’t give a rats ass what I look like, I just want my life back.
Trish and Christine, Doing extensive research I have come to the conclusion that NO surgeon that I have found will take insurance, they want the cash before surgery. Even when the explant surgery is covered they will not take the time or small effort to do the paper work. On their web sites they will say they take insurance or help you in filing, but during the meeting that is only if you have breast cancer. They obviously get paid well by the insurance companies for breast cancer surgery. Furthermore several surgeons will not return the implants to you even if you ask. I have discovered that if the implants are returned to the manufacturer the doctors get either free implants or money. In summary the breasts implant business is a dirty business from the FDA, manufacturers and including the surgeons. False claims to outright lies deception and misdirection all along the chain. I really do feel sorry for the women of America and the world who suffer from Bii and the greedy surgeons. Sorry for my rant, but I also have a severe problem with 25 year old implants that have caused my life to deteriorate to the extreme.
Oh my goodness Katie. 🙏🏼You poor soul. You sound like me except 10 times worst. I am considering remo inf mine the more I hear stories and observing how I’ve felt the past 3 years. Wishing you the very best.
Hi Katie. I am going through the exact same thing. My company just moved into a new building and I had a deadly allergic reaction to whatever is in that building. I’ve been to allergists/immunologists and he can’t find what’s wrong with me. I’ve been sick for quite a few years with bizarre symptoms. I got my saline breast implants in 2006. Was diagnosed with hypothyroidism in 2007 and gall bladder disease in 2008. In the last 5 years i have had stomach issues, weight gain, hair loss, elevated liver enzymes,severe digestion issues. This latest issue has me working from home and my company is giving me all kinds of grief. I can’t walk back in that office, I will die. The latest thing is that the allergist/immunologist noticed that my IGG, IGM and IGA levels were really low. He was thinking i had CVID (Common Variable Immunodeficiency), but after several tests he was not able to diagnose it definitively, but did say I may be on my way to having it. That woke me up. I’m getting these implants out. As soon as humanly possible. Good luck with your surgery! I’ll repost and let everyone know how I did after mine:)
Katie
Concerned husband here in total shock! Want to thank you for the path my wife will be taking. First thing checking insurance policy to see coverage to remove the implants. And hope for a happy healthier wife!!!!!! Chances for her figure to be restored ?????? What do I do for her ???? How many options are available for her to regain her figure?????
Thank you so much!!!!! Pete
Any update yet? Have you had them removed? I’m having so many problems and like you, stumbled across an article on BII. I’m dying to know if it helped you? Breast implants are a hard thing to let go of. For some reason I feel as if I will be less of a women if I remove them. So stupid!
Do symptoms start small? I got my implants in May this year. Shortly after I started experiencing joint pain, and now it’s hand pain, mostly in just one hand but sometimes two. I’ve identified the triggering movements and try not do them but it’s hard, they still hurt, and it’s not going away. Saw a hand dr and he says it’s tendonitis. He wants me to try therapy. I suppose it could also be caused by computer work that I do(even though it’s part time and certainly not all day)? My hips ache sometimes too, which is a new phenomenon, but I’m 42… this could all just be getting older?? My fiancé is not supportive, he loves the new boobs & is very skeptical of this illness when doctors don’t support it and all of my info is coming from the internet. I admit I’ll miss my shape but I’m tired of feeling bad and don’t want it to get worse.
Hi Allie,
They can start out small and gradually build up. Joint pain is a very common symptom of breast implants, it tends to improve and at times reverse with explant. The earlier women explant the better, the less symptoms they develop and the faster they can start healing. If you did not have symptoms before implants and you start shortly after, it is wise to make the connection. Many women have tried slowing down their work schedules, even quitting work for periods, adjusting their diets, doing various health treatments, etc. to only experience more symptoms in the long term until the implants are removed. The simple idea is to attribute symptoms to external stress, but the truth lies in the internal stress from the implants adding strain to the body and its various systems (immune, endocrine, neurological, metabolic, digestive). The foreign body reaction is an inflammatory immune response and in addition implants leach toxins (heavy metals, silicone, and chemicals) into the body.
Here is a wonderful resource for significant others to consider: “Breast Implants, A Husband’s Perspective”
Yes. Mine started with two symptoms – fatigue and brain fog. then over the years it got worse and worse with more symptoms appearing each year. This is my story: http://christinasfitness.com/ugly-truth-breast-implants/
I had breast implants in 200. In 2004 I was diagnosed with rheumatoid arthritis. In 2017 I spoke with a doctor in Dallas who told me to research ASIA syndrome seen many women who had autoimmune diseases after implants. He removed my implants and my RA has dramatically improved. Here is an article to read: https://www.the-rheumatologist.org/article/asia-a-new-way-to-put-the-puzzle-together/
Hi Carla,
Thank you for mentioning ASIA and sharing your RA improvement after explant! Dr. Yehuda Schoenfeld has done a lot of research on Autoimmune/Inflammatory Syndrome Induced by Adjuvants (ASIA), more of his articles can be found under Resources and Expert Researchers.
Recent large epidemiological studies have found associations between silicone breast implants and higher rates of autoimmune disorders.
In September 2018, the FDA released US FDA Breast Implant Postapproval Studies, the largest and longest study ever published of breast implant outcomes. Results indicated silicone breast implants are associated with higher rates of autoimmune disorders such as Sjögren’s syndrome, scleroderma, rheumatoid arthritis, stillbirths and melanoma cancers.
In October 2018, Dr. Schoenfeld published ‘Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis‘ which also found an association between silicone breast implants and a higher likelihood of various autoimmune/rheumatic diagnoses, including Sjögren’s syndrome, sarcoidosis, and systemic sclerosis.
Hello, have only silicone implants shown the risk of auto immune diseases, especially rheumatoid arthritis. Or are saline implants also risk for RA?
Who is the Dr in Dallas? I’m in Fort Worth.
Im blown away by all of this… Ive had my implants redone 3x because my body just didn’t like them at all. The last reconstruction redo was in 2016, and I have been to every freaking doctor I can see because of headaches, fatigue, weight gain, swollen and painful joints, dry skin, you name it. I recently had blood work done and was advised that I need to see a rheumatologist. Been there and done that. Now I see that I’m not crazy and I’m not a hypochondriac. But my fear of being 40 and having NO breasts is quite paralyzing. What are the alternatives to reconstruction? Did you all just remove the implants and just accept the concave and scared chest as is?
I’m looking into getting my removed as well. I’m also in my 40’s. They can do a fat transfer where they remove fat from other parts of your body so they can make your breast look natural and have shape so you shouldn’t have a concave. You can also get a lift at the same time. This is at least what I’ve found out through my research. I’m scheduling a consult very soon to get them out. Hope this helps some.
A friend had a successful reconstruction using muscles from her stomach. Just an option to consider.
Yes I just had mine removed I had mine for about 30 yrs I have went through hell now I know why could get know help with the money to get them removed loss of hair moody hand and neck and back pain for years as soon as they were removed I finally have a lot of release of the mystery pain I will not have them replaced ever I can’t belive I was told the saline were very safe I started only about 5 yrs ago notice something was really wrong and fill better knowingly I was not crazy !,
,
I had my final saline breast implant surgery in 2013, cosmetic reasons. I have been so sick since 2014. I have been to numerous specialists several times over these last 4 years including rheumatologist, neurologist, Endocrinologist, Spine. Numerous scans, ultrasounds and MRIs, I feel like I’m dying a slow death. I recently traveled to the Mayo Clinic in Rochester Minnesota twice this summer, begging for someone to help me. Note I live in Tennessee. Received another confirmed diagnosis of fibromyalgia. Since this past Monday my left breast has become so painful, swollen, lymph nodes enlarged from neck to axilla. Before marked pain and swelling had set in my Australian Shepherd started acting very odd. She jumped up on my bed and was fixated on my left breast, trying to open my robe with her nose. She repeated the following day. For the last 2 days shes been avoiding me… I feel like I’m losing my mind. Seen my gynecologist this Thursday , then ultrasound on Friday. Radiology reported prominent lymph nodes and benign simple cyst. I am begging someone to listen to me, I am sick! I am 49 years old, I have good insurance. I am pleading that someone please help me.
I have had double lumen implants in since 1989! In the last 4 years my health is deteriorating despite in the last 18 months doing everything perfectly to regain it. I have more and more “flare-ups” of unexplained nerve pain in my legs, hot flashes/clammy sweats, headaches, malaise, insomnia… Lately my right breast hurts. I feel worse for days after taking an infra-red sauna (due to temps making the implants melt??) I FINALLY have pulled my head out of the sand and think it’s these implants that are starting to take over and cause my decline. I want to go back to work full-time in Feb/March as I am about to be an empty nester but I need to feel better wo these periods of feeling like crap. Most of these top docs are books for 6 months+.. I can’t take 2+ weeks off from work right after I start…. I am in NJ… Anyway, I fear my implants have been in for soooo long that I wonder if it’s too late to regain my health anyway… Very confused and regretful that I ever got implants…
where are you in NJ?
there are women that have had them for 45 years, getting them out will change everything
Hello everyone,
I would like to start out by saying how happy I am to have found this site. I, like a lot of you on here have been having a slew of these symptoms that have recently worsened, most worrisome symptoms including breast pain, difficulty swallowing, heart palpitations, extreme fatigue, brain fog and vertigo so bad that I feel like I’m going to fall over. I literally have had to pull over while driving because I feel so out of it and it’s happening more and more. Something is not right with my body, I can just tell. My coworkers have expressed concern and they don’t know I have implants. I could just be self diagnosing myself but I’m convinced the implants are contributing. Any advice on first steps? Is it best to set up a consultation with a plastic surgeon, OBGYN, primary care provider (PCP) or maybe all three? I’m just worried not all PCP’s may be familiar with the symptoms of BII. A few years back I had blood work and my thyroid tested to explain my fatigue and it all came up normal. Any advice would help. I’m embarrassed to say I have had my silicone implants for 20 years with out ever having them checked. I’m not person who stays up on my annuals and I should know better, I work in the medical field as a RN and have great insurance. Can I also add on a lighter note, once these things are out (and healed) I’m going to sleep on my stomach, do push ups and chest exercises with out being paranoid I’m going to pop my implant and most importantly start loving my body for what it is! Any advice on just where to get started or stories on any explanting experiences would be helpful! Does anyone know how long you are laid up after implants are removed?
A lot of women on youtube are beginning to talk about their experiences from explanting their are also Facebook groups where people can give you information about their experiences explanting
Nicole I had my implants removed around October 29th 2018 today is Nov 9 the and I will tell you I fill so much better my right implant was the worst I have a indent on my chest from the implant pressing down on the chest wall I had my implants for about 30 yrs I was told at that time I would have them for life I had no problem in the beginning then I started to have strange illness no one could pin point mine were bad when they took them out I had drain tube that was fine the healing process took about 6 weeks I went back to work Dec 10 no lifting no reaching up for awhile you will be OK all I can say I wish I had not waited until 2018 I wish I had done it in 2017 I think it was the unknown and the money to do it it has opened my eyes knowing now why I had strange health problems go for it !! I think it will change your life bobs are not worth the harm on your mind an body Good Luck
very concerned I may have this illness and need to know who I can contact in Kentucky. Also, I am a breast cancer survivor and this is why I have the textured silicone breast implants. Has anyone else had this experience.
Hi Iletta,
There are other reconstruction patients who have experienced this. The National Center for Health Research has a list of Personal Stories, scroll down to “Reconstruction Patients” and you can click on individual names to expand their stories. There is also a Breast Implant Illness and Breast Cancer Survivor FB group.
Hi i would like to transfer the content of this website to arabic due to the lack of information about breast implant illness in arabic searches in google and instead of creating another website like this one i volunteer to pay for translation cost and send it to you and you can add the arabic language to the language options in your website i cant translate all the content at once because it cost a lot so i can translate the most important parts this is will be helpful for millions of women who has symptoms and they dont know what are the causes due to the lack of information’s if you are interested please email me.
Hi admin do you have an email? I have a question?
Hi Cara,
[email protected]
Is there any symptoms that are present with saine implants? I have been having symptoms for years and have just put two and two together to but I don’t want to jump the gun… I just need information on the saline part of it.
Hi Kat,
For examples of symptoms reported by women with saline implants, see Mentor Saline and scroll down to FDA Adverse Event Reports (they are from 2017 and some from 2018). Additionally, see Saline Breast Implants and Mold for FDA Adverse Event Reports that include symptoms women experienced with moldy saline implants.
Here are some examples of the first five Adverse Event Reports listed on Mentor Saline:
Adrenal fatigue, hypothyroidism, hashimotos, back and shoulder pain on left side, lower back pain – left side, heart palpitations. Link.
Chronic fatigue, body pain, brain fog, dry eyes. Link.
Mentor saline breast implants caused hashimotos and sjogrens auto immune. Hair loss, fatigue, brain fog, weight gain. Link.
Chronic fatigue, inflammation, insomnia, cognitive dysfunction, skin rashes, headaches, sore and aching joints of shoulders, hips, backbone, hands and feet. Anxiety, depression, and panic attacks, pain and/or burning sensation around implant and/or underarm, cold and discolored limbs, hands and feet, general chest discomfort. Shortness of breath, frequent urination, numbness/tingling sensations in upper and lower limbs, ear ringing, sudden food intolerance and allergies, vertigo. Link.
This was the month and year i started having heart palpitations, inability to exercise, chronic fatigue, numbness, neck and back pain, frequent fevers, anxiety, lightheadedness, profound muscle weakness, couldn’t recover from anything, feeling like i was literally going to die. Extreme endocrine (blood sugar fluctuations). Inability to sweat. Heat and cold intolerance. Rashes and sensitivities to any lotions, perfumes. Link.
Here is an example of a FDA Adverse Event Report listed on Saline Breast Implants and Mold:
It was reported that a female patient was implanted with mentor smooth saline breast implants in 2002. She stated that her immune system was getting worse since implantation as she experienced autoimmune symptoms. She also reports headaches, brain fog, joint pain, body aches, chronic fatigue, dry eyes, weight loss, digestive system issues, sore feet, and increased recovery period from any surgery. She claimed that the implants were removed in 2016. The implants were found with faulty valves and mold. She also claimed that the implants caused harm to her children during pregnancy and breastfeeding as her children’s immune system were over-reactive to everything. Link.
Hello. I have been researching this for a year now and planned to have the procedure next month. However I have discovered this page and have been reading all of the claims against Mentor saline and silicone implants. My question is, why are all of the reported days feb-oct 2017? Why are they not more spread out than that? Just trying to make sure I’m not reading skewed info.
Hi Liz,
The FDA MAUDE database has breast implant related Adverse Event Reports for the last 10 years. Reports come up from as early as 2008 for Mentor and Allergan breast implants. For efficiency, I compiled only recent ones from 2017 and 2018 on the Mentor Saline page. You can search for more reports in the MAUDE database simply by entering a date range and manufacturer. To note, in the last year, as awareness efforts and media attention have increased, so have the number of reports. It is a voluntary reporting system therefore due to increased awareness of breast implants causing illness and how to report to the FDA (MedWatch Form), the volume of reports have recently significantly increased.
I was going to have the surgery dec 11th and I joined 3 different breast implant illness groups totaling with 70,000 I’ll women. I am not rescheduling surgery
Liz I had my implants in 1984 they had recalls on the dowel corning at the time I was not having problems I was included in the recall didn’t think it would affect me I was fine then I started having strange illness went to Dr’s they made me fill like I was crazy I started to think I was going crazy I was 25 yrs of age I thought what the hell is going on with my body neck pain back pain thought I was losing my mind my joints and bones started to hurt real bad have them taken out I think we are getting a lot of information now we are not alone in this I would rather have small bobs and a a better quality of life I’m not that old to fill so crazy lol
I have sailine also and just learned about BII because I’ve had symptoms for years. My implants are almost 16 years old. Keep reading. You will find that saline is no safer than silicone. Watch videos on YouTube.
Hi there,
I am so happy I found this website. I have been sick for 19 years. I just keep getting progressively worse. I am desperate to have an explant. My plastic surgeon is deceased. How can I get my medical records? I was implanted first time in 1994 and one leaked and reimplanted both in 1997. (Mentor)
Thank you
Hi Cynthia,
Do you have saline or silicone implants? If you have silicone and were implanted between 1992-2006, during the moratorium, then you should have been placed in a study. There was a lady in the breast implant illness groups who had luck with contacting Mentor and found out information in regards to the study she was enrolled in and her implants. She called the Mentor Cincinnati office (now Ethicon, a subsidiary of Johnson & Johnson) and spoke with a clinical study administrator. The contact for that office (Ethicon in Cincinnati) is 513-337-7000, you can try to call and ask to speak with a clinical team/study administrator.
See also Medical Records Disposition When Closing a Practice and Requesting Medical Records.
By the way ladies, since my stage 3 Br Ca dx 6 yrs ago, The MOST important lesson I’ve learned is that each girl/ woman MUST be her own advocate. There are NUMEROUS factors in our daily lives that are contributing to BR CA. Women’s healthcare has not progressed to where it should be in 2018. Most Ob-Gyns don’t have a clue about how to balance hormones. And until I came across this site, NO doctor suggested my symptoms might be related to my one remaining breast implant. PLEASE do not stop until you find a doctor who will listen to you!! Thank you ALL for posting your experiences! Women must continue to support each other more now than ever.
I am a 41 year old female who had Mentor breast implants done 7 years ago. I was previously in perfect health, extremely active, won 5k races, etc. After 5 years of having implants my health slowly started deteriorating. I now have extreme fatigue, brain fog, joint pain throughout most joints in my body, back pain, tendon pain, plantar fasciitis, gastrointestinal issues, severe dry eyes and mouth, difficulties with temperature regulation, light headed, extreme sensitivity to sunlight etc… .. I can no longer workout to the level I use to . I don’t feel like the same person. LOOKS ARE NOT WORTH JEOPARDIZING YOUR HEALTH!! I wish i never had breast implants. I’m getting them removed soon.
I just found out about all of this and am so scared.I got my Mcghan silicone implants style 80 smooth in 1988.I never have had any problem until about 4 years ago when i started with allergies,stomach issues,aches,pains,anxiety,depression,mood swings,eye twitches,ringing in my ears,brain fog….and lately i started having neck and shoulder pain and a tightness on my right side in my chest when i take a breath.It almost feels like something is sitting in there.I am so glad i came across this implant illness and now i am the process of finding an explant surgeon.I live in Illinois and would love referrals,but am willing to go anywhere to get the OUT.
.I am afraid i have had them so long and am 66 that this could be a dangerous operation.I have always been healthy and active and take great care of myself.Lately i have had so many things happening within my body i was beginning to think it was me.Now i have something to work with.
I had consultation with plastic surgeon for left breast explant. I have numerous symptoms including Lymphedema symptoms. I have Lymphedema in right arm from Mastectomy 6 yrs ago. I asked to have a post op drain & surgeon said that drain was not necessary & “they” don’t use drains just to do explant. I insisted that I have a drain. Are drains not typically used for explants?
Hi WJ,
The majority of surgeons do use drains for explant, however using drains post-surgery is a surgeon preference. In both cases, ladies have healed well. Pros: drains are primarily used to collect excess fluid that may accumulate which can prevent seromas (pocket of fluid buildup), infection (fluid buildup can be a breeding ground for bacteria), hematoma (a buildup of blood if there was excessive bleeding during the surgery), swelling and pain (fluid buildup can cause pressure on the incision site and to adjacent blood vessels, nerves, etc). Cons: they may increase risk of infection the longer they are in you, because they can be a pathway for bacteria to get inside. Fluid buildup can result from how complicated a surgery is, such as if there is an infection, more surgical trauma (if there is a lot of surgical dissection, if there is excessive bleeding, etc.), or a lot of dead space (area where the implants previously were). The top explant surgeons generally do use drains to help with fluid accumulation, see Drains in Explantation Surgery by Dr. Chun on YouTube. Ladies have healed well without the use of drains as well.
A website with a lot of information!
I would be proud to be listed with my work. We investigated a patient and could proof that silicon can migrate throught the body. Thanks and wish you lot of success, Henry Dijkman
Kappel et al. Clin Med Rev Case Rep 2016, 3:087
ISSN: 2378-3656
ClinMed International Library
Citation: Kappel RM, Boer LL, Dijkman H (2016) Gel Bleed and Rupture of Silicone Breast Implants Investigated by Light-, Electron Microscopy and Energy Dispersive X-ray Analysis of Internal Organs and Nervous Tissue. Clin Med Rev Case Rep 3:087 Received: December 29, 2015: Accepted: February 06, 2016: Published: February 09, 2016 Copyright: © 2016 Kappel RM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Volume 3 | Issue 1
Gel Bleed and Rupture of Silicone Breast Implants Investigated by Light-, Electron Microscopy and Energy Dispersive X-ray Analysis of Internal Organs and Nervous Tissue R.M. Kappel1, L.L. Boer2 and H. Dijkman2* 1Plastic & reconstructive surgeon, Dr. Kappel Institute, Zwolle, The Netherlands 2Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands *Corresponding author: H.B.P.M Dijkman, Department of Pathology, Radboud University Nijmegen Medical Centre, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands, Tel: 31-24-3655297, E-mail: [email protected]
Abstract Objective: We studied a patient who died in 2008 at the age of 56 and had been exposed to gel bleed from her silicone breast implants for 17 years. Tissue samples and nervous tissue could be obtained for analysis.
Design: During autopsy, a wide range of different tissue samples were collected, frozen and embedded in paraffin and plastic (Epon). The paraffin samples were stained with Hematoxylin and Eosin (HE) as well as with Modified Oil O Red (MORO). Tissues embedded in plastic (Epon) were sectioned and prepared for light microscopy using toluïdin blue staining for Transmission electron microscopy (TEM) and Energy Dispersive X-ray microanalysis (EDX) to measure elemental Silicon (Si).
Results: We found 2 types of silicone material in multiple tissue and brain samples of this patient. The first is a droplet-like form. EDX measurements demonstrated that the droplets are composed of elemental Si. The second is a plaque-like form; these structures are comprised of elemental Si and Ti (Titanium). Occasionally we found that these plaques were located inside the tissue without a lining and sometimes they were located inside the lumen of blood vessels.
Conclusions: The use of EDX analysis over light microscopic examination only, is now a contributing factor for the establishment of silicone bleeding and migration throughout the whole body in high amounts. Keywords Silicone breast implants, Silicone gel bleed, PDMS, Si, TEM, EDX
Hi Doctor. Do you have any studies on saline implants? I’m located in Michigan. Do you have any colleagues that are involved in breast implant research in Michigan (USA)? I believe that my symptoms of CVID are directly related to my saline breast implants. Any help you can provide is appreciated. Thanks.
This website has been SUCH an amazing resource! Thank you for everything you do. You should really link up with Christina Roulund-Dennis, I discovered her blog post on breast implants and how they dont have to be leaking to get sick (http://christinasfitness.com/your-implants-dont-have-to-be-leaking-for-you-to-get-sick/). She answered all my questions on Facebook chat for free and has helped me immensely. You two should team up!!
I had 335cc silicone implants placed in April 2016. As a bikini competitor I felt I needed them in order to be competitive nationally in the sport, despite consistently placing top 2 without them. In March of 2017 I started experiencing extreme fatigue. I went from lifting weights 6 days a week to barely making it to the gym 1-2 times. I had some basic blood work done which revealed low estrogen so I was started on oral birth control again (had been off for a few months). Also had the ANA test done which was negative at the time. A year later still with chronic fatigue and now also dry eyes, memory issues, and “brain fog” people describe. I went back to my doctor and broke down in tears, a shell of my former active self. I contributed it to working overtime, planning a wedding, etc.. She ordered more tests. This time my ANA came back positive, along with low WBC’s, indicative of an autoimmune or connective tissue disease. I have an appointment with a rheumatologist in 2 months (soonest opening), but deep down I feel it is the implants that are the root of the issue. No one in my family has a history of lupus or anything autoimmune. After reading copious articles and other women’s stories, I feel validated. I also feel angry that my doctor never even discussed or mentioned the autoimmune factor. But why would he? There I was with $6500 in hand ready to spend. I should have done my own due diligence first but I had no idea that anyone could get sick from implants, it was never mentioned in any consult. Next I plan to do the heavy metals testing and ask for an ultrasound to see if there is rupture, I have Aetna insurance so I am also going to start the process of trying to get approval for removal. Looking forward to getting my life back! Thank you for all of the great resources on this site!
Hi. Do you have data that quantifies the incidence of illness, rupture, and other ill effects? Our daughter wants implants and we hate the idea. She does respect data, however, much more than she respects her parents.
Thank you
Hello I’m a reporter and I’m working on a story on breast implant illness. Is there a way to reach the admin of this site directly?
Hi Emily,
Thank you for your interest in the news story, please email directly [email protected]
I have a high school friend that recently had an explant surgery. She has been very forthcoming with us all and sharing her story on facebook.
It was a long process of determining that it was her implants and as she says now she should have seen the signs, they were all there, but like most
of us, we attribute our symptoms to other things. She is still in recovery process at home but I am sure that she wouldn’t mind sharing her story.
Can you recommend a surgeon to remove silicone breasts implants in the Durham, NC area?
Hi Brenda,
Check out the Explant Surgeons page. Dr. Glenn Lyle seems popular in North Carolina, he’s been doing increasingly more explants of recent. There is also a Breast Implant Illness Carolinas FB Support Group where you can research local surgeons in that region.
Hi, my friend is very concerned about her having an illness relating to her breast implants (Mentor). I was wondering if there are any studies proving the above symptoms are caused by breast implants? Thanks.
Hi B,
See the pages below for references to studies showing the causative effects of breast implants.
Scientific Articles
Dr. Arthur Brawer, in particular see “Chronology of systemic disease development in 300 symptomatic recipients of silicone gel-filled breast implants.” He states: “The findings in this study strengthen the conclusions of other investigators stating that silicone gel-filled breast implants are the direct cause of a novel systemic illness.”
Expert Researchers
Resources, see Educational Links
Safety
Additionally, Dr. Victor Urzola has a current ongoing database where he is conducting a research study on his explant patients. In 2017, he stated:
“Over the past year and 8 months I have learned and researched a lot about this condition. After explanting over 100 patients and seeing the extraordinary post operative reports with over 85% of patients reporting complete remission of their symptoms or at least an important improvement, we are committed to starting a scientific investigation with the purpose of validating BII as a syndrome and getting the medical community to recognize it as a problem affecting thousands of women around the world.”
When all these men came in symtoms that were similar (young and old) to their doctors. and hospitals, eventually they were taken seriously when there was a dialogue started with real doctors than researchers in the US and France….. The problem, here No profit to me made by taking these cash cows off the market, “various types of Implants” for cosmetic reasons, that make so much $$$ for the the manufacturers and the surgeons.
I wanted to take a moment and share my experience in hopes that it would help someone else get through this terrible real disease known as implant sickness! I have been sick and in pain for the past year and as I went from doctor to doctor and no one seemed to believe me when I expressed that I felt my symptoms were being caused by my implants. My joints had a burning sensation of pain, I was constantly fatigued. My hands and feet were always swollen and in tingling pain. I just felt an unusual daily fogginess that was affecting my life. I was recommended to go see Dr. Kevin Brenner by a friend of mine who had her implants removed by him 2 years prior for similar reasons. I was very hesitant at first because I had been pushed away by 3 other doctors whom in the nicest way possible basically told me how I was feeling was all psychological when I knew it wasn’t! However, because my friend spoke so highly of her experience I figured I had nothing to lose.
Dr. Brenner and I met for a consultation 6 weeks ago and he spent over an hour with me listening to my real health issues and explaining his experiences with patients with similar symptoms. It was the first time in a long time I didn’t feel crazy for how I was feeling. I am now 1 month out from surgery and practically in tears writing this comment because I feel like myself again. I am disappointed that the other doctors didn’t take my illnesses seriously because I could have had this relief a year ago. I am so grateful that I was introduced to Dr. Brenner. He understood me, didn’t judge me and although my only concern was feeling healthy, I am so happy with the way my breast looks now without an implant and just a lift.
Point being breast implant illness is real and I am proof of it and to anyone else who is experiencing what I did- don’t give up!!There are doctors out there like Dr. Brenner that are knowledgeable and willing to help! I am so grateful for my life back!
Hi Megan..So glad you are feeling better.. Where is Dr Brenner located ?? And what’s his full name please..
Thank you for your story! I have been feeling like the that crazy lady that has all these symptoms that no doctor can figure out what is going on with her. I have gone to many many doctors and today I went into a different doctor and told him that it might be my implants, and they looked at me with a surprising face asking me, well does the breast hurt? when I was giving him all the other symptoms associated to breast implant illness. I have ♦Fatigue ♦Weakness ♦Aches ♦Muscle Cramps ♦Unusual Pain ♦Ice Pick Pain ♦Headache ♦Sinus Problems ♦Cough ♦Shortness of Breath ♦Joint Pain ♦Morning Stiffness ♦Memory Issues ♦Focus/Concentration Issues ♦Word Recollection Issues ♦Decreased Learning of New Knowledge ♦Confusion ♦Disorientation ♦Mood Swings and all,they keep telling me its fibromyalgia or chronic fatigue syndrome, or myofascial syndrome…At this point, I have no idea where to turn to or what doctor will tell me that it is medically necessary to take them out so my insurance will cover the cost. Any suggestions on where to go in the Portland Oregon area would be appreciated
@Megan: Thank you for sharing your story. I had right mastectomy in 2012 for stage 3 Br Ca. I still have left saline implant in since 2005. Over the past year and a half, my symptoms have been increasing. Very similar to yours…pain, face,neck & arm swelling, numbness & tingling in left arm, migraines, abdominal swelling, etc. All tests are negative. Numerous Dr opinions told me it was all post chemo/tad related. I’m a former Oncology/chemo RN. So I knew that explanation just didn’t feel right for me. Finally, a few weeks ago I started researching breast implant illness & reading personal experiences of others. I have a consultation at UNC to have implant removed ASAP.
please can we get the full information for Dr. Brenner?
I feel as though I just woke up. I have had breast implants for 28 years (March of 1990). I never had a problem until 2006 at which time I thought something had happened however, my surgeon said I must have just pulled a muscle and that the implants seemed fine. Now that surgeon is old and the shop is closed up. I have been suffering for the past 13 years with arthritis, fatigue, brain fog, inflammation, hormone imbalances, and adrenal fatigue. My naturopath had me do SO many tests which kept coming up negative. Gluten free/dairy free for 12 years and recently have removed all lectins. Yes, I am 60 but have always been active, eat incredibly clean, don’t take any meds albeit herbs and supplements. Why then do I always have pain? Duh!! Needless to say I am a bit freaked out right now. I love my beasts and always thought it was the right thing to do way back then. After reading most of these posts I suppose I really have only one option. Is there a forum from women that have had explants. The reason I did the implants (I’m sure like so many others) was the result of nursing three kids for several years. Support on the new journey would be terrific.
Hi Jacquelyn,
Very glad you found this information, you now have the knowledge and empowerment! It is a profound realization and defining moment when one connects their breast implants to their declining health journey. There are over 160+ breast implant illness support and awareness, groups and pages on Facebook where women share their experiences, see here for a list.
Jacquelyn, I am in the exact same spot as you. I got my implants in 1989 and in the last 4 years my health is declining. I need to get these implants out asap! Did you get yours out?
Jacquelyn, Your words “I feel as though I just woke up” made me want to reply. I am also, in my 60’s, had implants 20 years ago because of sagging boobs, eat clean, gluten free/dairy free, etc. My naturopath is who has been encouraging me to explant because of all of the symptoms I thought were normal and natural. I now have a date for the explant and can not wait for March 19th.
I have the gummy bear saline implants and i have spent the past 4 years trying to figure out what autoimmune disease i have that is making me constantly sick, in pain, fatigued and just all over sick feeling. After finding this page i believe my implants have to be the cause!! Does anyone know if medicaid will cover this procedure? I saw in a previous post that medicare will if coded correctly so i wondered if medicaid was the same
So you are aying Medicare will remove implants due to Illness
Hi Anita,
Medicare and Medicaid will often cover the costs of breast implant removal.
According to Centers for Medicare & Medicaid Services (CMS), which is the government program that regulates Medicare services, Medicare authorizes the removal of breast implants for the following reasons:
2. Removal of BREAST IMPLANTs
For a patient who has had an implant(s) placed for reconstructive or cosmetic purposes, Medicare considers treatment of any one or more of the following conditions to be medically necessary:
•Broken or failed implant
•Infection
•Implant extrusion
•Siliconoma or granuloma
•Interference with diagnosis of breast cancer
•Painful capsular contracture with disfigurement
Source: Cms.gov Medicare Coverage Database
I have Saline implants (Mentors) and for some years… I have been suffering from Fibromlgia for yrs now some numbness on the left side just thigh on occasions..depession, hair loss badly, and alot of other symtons as others have mentioned…I am on SSI with Medicare…So, you are saying Medicare will pay for the removal of these harmful implants… The pain is sometims so bad…… I saw a news broadcast on tv of a news comentator suffereing from Breast Implant Illness..
I have been chronically exposed to mold over the last 6 years. I had my implant replaced in 2015. I recently got extremeley ill and had to move out of my house. I slowly started getting better, I found a doctor who specializes in mold illness, the first thing he told me was to have them removed because mold can get behind the implants and colonize. My first question is has anyone ever heard of this and second could it happen in a matter of 2 years. I AM MENTALLY AND EMOTIONALLY SICK OVER THIS, I FEAR IT WILL CAUSE A DIVORCE AND TEAR MY FAMILY APART.
Moldy implants is REAL. I just saw a picture of moldy implants that were just removed on a practitioners group I belong to. I am very familiar with mold illness. Have them removed ASAP! Good luck to you.
Hello- I have just become aware of BII. I suffer most of the symptoms. I have no Insurance and funds are the only thing holding me back from removing my implants. Are there any programs that can help?
Hi Tara,
There is a charitable Explant Assistance Program but it is currently on hold as they go through a backlog of a heavy volume of applications they received after simplifying their requirements in January 2018. They offer up to $5,000 to help women with explant surgery with proof of record of implants/implant surgery and explant. If there are funds leftover the program will hopefully reopen at some point. It may be of use to periodically check in with them to see where they are at and in the meantime to request your medical records from your implanting surgeon.
Ladies please remove your implants regardless of how long you had them in. It doesn’t matter if you don’t have symptoms now, you will in the future so save yourself the suffering! I had my Mentor Memory Gel implants under the muscle for 8 years. I just got them explanted with en bloc 3 weeks ago. All my symptoms are 90% better and some are completely gone. I can’t believe it almost! For the past 2 years I was suffering with brain fog, joint pain, numbness and pain in my hands and feet, extreme thirst, hair loss and slow growing, fatigue, rashes, dry skin, muscle spasms in my neck and back. I was also diagnosed with fibromyalgia and scleroderma which I have an identical sister who has neither of these. I went from doctor to doctor trying to figure out what was wrong with me. I was completely healthy for years and then it was like a switch, I woke up one morning feeling tired and achy joints that productively got worse over the past 2 years. I wish someone would have shared their story with me 2 years ago. Up until 4 months ago, I’ve never heard of breast implant illness and that is why I am determined to spread the word. I had my explant by Melissa Johnson in Springfield Massachusetts. She removed my implants and capsules and I do recommend her. The next day after the surgery I noticed immediately that the constant muscle spasm in my neck and back was gone and over the next couple of days my brain fog cleared up, rash on my back and scalp went away. My skin had even regained it’s glow and shine. I started the detox process and in 6 months I will retake all bloodwork and hoping my autoimmune and centromore ab are normal. I pray that you all have the strength to get them removed and regain your health! Please share your stories with ANYONE!
I have have the same implants,under the muscle, got them in 2009, symptoms started in 2015, I’m so sick, and have a 3 yr old daughter to care for, I have every symptom. I was told forever it was anxiety, Then doctors started making up excuses not to see me like saying I have two insurances one from 1991, now I am in excruciating pain around the implants. I don’t know what to do!
Omg- I was diagnosed with the same autoimmune disease and have been so paralyzed with fear. How are you now?
I have mentor memory gel implants too..! This past summer I have been having joint pain and inflammation and I thought it was from Gluten but now I’m investigating explant surgery as my implants are 10 years old. Thank you for sharing your story!
Wow, this website is a godsend,
I’ve gotten every test under the sun & things keep coming back “healthy” yet I know that’s impossible considering I have most of you have described yet am exhausted all the time, have numb hands & toes, & have gotten RA, I had saline for 20 years since I was 16…. & am convinced I have BII
Can someone recommend an explanation surgeon in Canada, preferably Toronto.
Thank you so much & im wishing you all health & wellness.
I just spoke to a lady who went to Dr Schiller in Toronto. She has a FB page on this.
Hi all, I was diagnosed with D.I.S.H. at 40, but had very few symptoms and was able to exercise and walk daily until 2013 when I had silicone implants with a lift put in. It seems to have accelerated the bone deterioration and for the last year I have been in a fog, and with pain all the time. Also many of the other illnesses named here by others. Does anyone know if Medicare will pay for explant? I am also afraid I will go through all the expense and pain with no improvement in my health and ugly breast.
Hi Deborah,
Medicare often does pay for explant when the correct codes are used and the criteria apply. See insurance.
Breast implants are large foreign bodies that contain silicone, heavy metals, and other chemicals. They are toxic interference to the body and can exacerbate prior conditions, in addition to creating more symptoms. You can read explant healing stories under Life Since Explant Club and see a gallery of pictures on how the heal is real at BII Aware, under BII Photos. There are also many news stories of women’s healing testimonies under Resources.
Dr. Lu-Jean Feng has stated, “I would say that most patients recover their illness, recover their health after a proper explantation. Percentage? I would put it at over 75%.” (Webinar Transcript, pg. 7)
Last year, Dr. Victor Urzola stated: “Over the past year and 8 months I have learned and researched a lot about this condition. After explanting over 100 patients and seeing the extraordinary post operative reports with over 85% of patients reporting complete remission of their symptoms or at least an important improvement, we are committed to starting a scientific investigation with the purpose of validating BII as a syndrome and getting the medical community to recognize it as a problem affecting thousands of women around the world.”
In regards to breast aesthetics post explant – my breasts went back to how they were prior to implants, the scars have even faded. There are many beautiful results in the community and there are FB groups you can join to see pictures. Find an explant surgeon who is experienced in explant.
So I found this after googling why I can’t do push ups with a boob job. I had no idea BII existed and now I am freaked out. I am young and just had the implants put in 7 months ago. I was 32a-b before. I had 275cc gummy bear put in. I am a model and I am worried my breasts will look bad if I remove the implant now.
1. did anyone have an explant and have their boobs look like they previously did?
2. Since I only had it about a year ago will it be easier for my breasts to return to normal?
3. Did anyone have any procedures / laser to remove the scar?
4. I live in NYC if anyone can recommend an explant expert doctor!
Hi Marie,
There is a growing surge of models (Playboy, fitness, etc) and celebrities who have explanted and openly posted about BII, such as Crystal Hefner, Karen McDougal and other former Playboy models, Yolanda Hadid, Eden Sassoon, Nicola Robinson, and more. See Resources for news articles.
As well, there are fitness models and women explanting in the BeachBody fitness community. They have been able to improve their health and resume workouts after explant. SJ McShane is a fitness and BII advocate who did a cover story on the ugly side of breast implants. She states, “The fatigue became so bad I had to stop working out” and other symptoms that resolved post explant.
There are many beautiful explant results in the community. It is important to find an explant surgeon who has a lot of experience in explant. It seems the less time one has had them in, the faster women are recovering.
There is a NJ, PA & NY Breast Implant Illness FB support group where you can ask questions and see pictures.
Dr. Brian Buinewicz or “Dr. B” in Flemington, NJ and Doylestown, PA believes in BII and is a very good explant surgeon who comes highly recommended by women.
Hi,
I had breast implants fitted back in 2009. For the last year or so I’ve had days (around 4 days a week) where I’ve been in a tremendous amount of pain. To the point that putting on a bra hurts. I really want to have mine taken out but am unsure whether to go back to the surgeon/company I had them done with. Has anyone had pain related issues with their implants. I’ve not had fatigue or hair loss just a painful right breast.
Hi Victoria,
Yes, pain in the breasts is a symptom. It could be from capsular contracture where the capsules (scar tissue) encapsulating the implants harden. If you have textured implants you may want to consider doing the CD30 pathology test on the capsules to rule BIA-ALCL. Find an experienced explant surgeon who will do full capsule removal. A smaller percentage of women have gone back to their implanting surgeons but most seek out another surgeon who is experienced in explant.
Marie,
After 7 months of implants, I am sure your breasts will look the same as they were before the implants after removal. I had my implants for 8 years and my breasts look normal. I had my implants removed with Dr. Melissa Johnson in Springfield Massachusetts. She did a great job explanting my implants with en bloc and she put lots of glue on my incision so you can barely see it them. Whether you use her or choose another doctor, I suggest you get them removed. I was fine after my implants and then one day I just became sick, my hair fell out, joint pain, numbness in my hands and feet, brain fog, extreme fatigue, dry skin and muscle spasms in my neck and back. It’s only been 3 weeks and already my symptoms have improved 90% after 2 years of pain and suffering. I had no idea what was wrong with me. I have also been diagnosed with autoimmune, fibromyalgia and scleroderma. I am hoping after detox these will all go away as well. Save yourself from this and remove them before they harm you!
I’m so grateful for this website – thank you so much for continuing to gather thorough, science-backed information about breast implant illness and for building an extremely strong case against them. It certainly helped in my decision, although my gut and intuition were telling me they were poisoning me for years. I had my cohesive gel silicone implants removed two weeks ago and am still in recovery, but I feel great. A literal and figurative weight off my chest!
For anyone out there who is still trying to identify what’s making them sick but has an inkling that it could be their breast implants, IT’S THE BREAST IMPLANTS. I personally had saline implants put in in 2003 and then had a revision in 2012 and had the new, “safe” (NOT SAFE) cohesive gel silicone implants put in in the spring of 2012. By the fall of 2012 my hair was falling out, but I thought it was stress and depression. By spring of 2013 I got hair extensions to mask the hair loss. Then came exhaustion which built up over time and became so severe at some points (by 2015), I couldn’t even get out of bed to turn over the laundry. Other symptoms that worsened over time: brain fog, memory issues, dry mouth, dry eyes, night sweats, random auto-immune responses. Somehow I held down a crazy corporate job in NYC through out all of this. I attributed my issues to severe adrenal fatigue from my stressful job and Epstein-Barr (I had mono in high school and had read about the long-term effects of EBV). I did everything under the sun to become healthy. I changed my diet to eat totally clean, addressed parasites/candida/gut health, stopped drinking alcohol, became a serious meditator, cut out toxins from all of my beauty/cosmetics, regularly went to acupuncture, made sleep a priority, etc. I think this maybe slowed down some symptoms and masked them for a while and I maintained this for 3 years but eventually, IT STILL GOT WORSE. Then by the fall of 2016, the fatigue and brain fog was insane. I got a brain MRI, did a sleep apnea test, went to a brilliant immunologist, and got every single blood test under the sun, including ones for rare genetic diseases. Then I started to get sick – flus, viruses, pneumonia (multiple times in one year). I started randomly breaking out into hives. Not in reaction to anything I ate or put on my skin. I would just get hives. I became allergic to kombucha. I couldn’t stop thinking about how my immunologist had listed off five things that cause auto-immune disease and POISON was one of them. I just felt in my gut that my breast implants were poisoning me. I started researching and came across this website. I knew what I needed to do.
Thank you again for this site and all of the work you’re doing!
Hi RR,
Thank you for sharing your detailed story and important message on the toxicity of breast implants! This movement is largely driven by women raising awareness by speaking their experiences with breast implants. I am very grateful for each woman who is able to come forward.
You have described my situation almost exactly, please elaborate on what you did! Did you remove them? Did You regain your health? I’ve gotten every test under the sun & things keep coming back “healthy” yet I know that’s impossible considering I have most of you’ve described & am exhausted all the time. I have also had saline for 20 years since I was 16….
My apologies for not responding sooner – I didn’t check back here. Yes, I had the implants removed and my recovery has been amazing. Immediately – like just a few days after surgery – I felt BETTER than before the surgery even though I was obviously still recovering as surgery is a big trauma to the body. It made no logical sense but I think my body just immediately got to work in doing major healing. I also felt like myself again on a deep level. I never realized how much I felt the implants in my body, subconsciously, and what a relief it was to have them gone. It’s only been 3.5 months and my hair is finally growing back (a miracle), my skin is revived, the brain fog is gone (FINALLY), and overall I’m in perfect health. I’m still cycling through some detox periods where my body continues to eliminate the toxins it’s buried all over my body because it couldn’t manage the toxic load leaching from the implants over all of those years. Basically, I’ll have a few days of exhaustion and night sweats where my body does a sweep of detoxing, and then I bounce right back. It’s very different than the extreme, chronic ill health I was experiencing before. To those of you that are so sick and concerned that your body can’t handle a surgery, I totally shared that concern but the body is an AMAZING thing and literally by the next day I felt better than before. That was my experience, at least. Best of luck to all of you!
I love you, RR, for sharing your whole story. 🙂 I relate to your “before” and dream of your “after. You helped me finally get excited about surgery. Thank you!
how is your recovery going?
My apologies for not responding sooner – I didn’t check back here. Yes, I had the implants removed and my recovery has been amazing. Immediately – like just a few days after surgery – I felt BETTER than before the surgery even though I was obviously still recovering as surgery is a big trauma to the body. It made no logical sense but I think my body just immediately got to work in doing major healing. I also felt like myself again on a deep level. I never realized how much I felt the implants in my body, subconsciously, and what a relief it was to have them gone. It’s only been 3.5 months and my hair is finally growing back (a miracle), my skin is revived, the brain fog is gone (FINALLY), and overall I’m in perfect health. I’m still cycling through some detox periods where my body continues to eliminate the toxins it’s buried all over my body because it couldn’t manage the toxic load leaching from the implants over all of those years. Basically, I’ll have a few days of exhaustion and night sweats where my body does a sweep of detoxing, and then I bounce right back. It’s very different than the extreme, chronic ill health I was experiencing before. To those of you that are so sick and concerned that your body can’t handle a surgery, I totally shared that concern but the body is an AMAZING thing and literally by the next day I felt better than before. That was my experience, at least. Best of luck to all of you!
I live here in colorado springs and I am having several symptoms. Do you know of any doctors here that you can recommend?
Hi Kelli,
Dr. Linda Huang in Denver, CO is a good explant surgeon. She has been doing explants for the past 20+ years and recognizes that women are getting better after explants. There have been many happy women who have recommended her. There is a Colorado Breast Implant Illness FB Support Group where you can engage with other ladies in that region who are exploring explant surgeons and going through explantation, you can ask questions and see pictures.
Hi, I have silicone gel implants i’d like to remove as well and i called Dr Linda Huang’s office but i was told that she charges 8k for explant which is double the price of most surgeons around here. Do you have any other dr who can just as well and doesn’t charge that much?
Im in NY.C Id love to chat with another like me. I got lupus, hidrdeitis and a collapsed back from this dumb implant. Email me at [email protected] Lynda
Hi Lynda,
There is a New Jersey, New York, and Pennsylvania Breast Implant Illness FB Support Group where you can chat with other women. There are also larger and more general breast implant illness Facebook groups and some surgeon specific Facebook groups. See here for a fuller list of 160+ Facebook support and awareness, groups and pages.
Hi, I am a male, years ago I got a silicone chin implant to enhance the look of my chin. Since then I have had many health problems that doctors can not figure out. The plastic surgeon says it is impossible that such a small solid piece of silicone in my chin could cause health problems. What do you think?
Hi David,
Silicone toxicity, foreign body reactions, and biofilm can develop with a variety of implants. The immune system reacts to silicone. Dr. Douglas Shanklin (pathologist) and Dr. David Smalley (immunologist) did a lot of research on the biochemistry/immunology of silicone, their articles are listed under Expert Researchers. One of their publications, “The immunopathology of siliconosis” states “Recent evidence confirms the fundamental involvement of the human immune system in the reaction to implantation of silicone-based medical devices….The clinical consequences of siliconosis are common and can be severe in some individuals implanted with silicone devices.” You might be interested in the Silicone Hypersensitivity Panel, it measures lymphocyte (white blood cell) sensitivity to silicone. There is a lady who had her chin implant removed and it gave her body a chance to heal. There have also been other cases of implants being removed in the community due to similar symptoms as breast implants. In the presence of unresolved health complications, removing foreign material from the body is an important step towards healing.
38 yrs old, saline implants for nearly 13 years. I have a ton of the symptoms but the last 5 years have been rough and last year REALLY rough. blood test are normal except for progesterone low- got on meds and felt much better. then 6 months in it hit me again. I always had minor things in between here. My mom stumbled upon and read these stories and called me to say- THIS IS IT! this is what is wrong. I always tried to chalk it up to my hormones( I have PCOS) but knew something was not right! when it hits hard it gets hard to breathe, I feel like I’m in a cloud, lights hurt my eyes(even without headache sometimes- I wear sunglasses while sitting at my desk a lot), my hands and feet, sometimes arms and legs get numb/tingly/cold/sweaty, my words don’t come out right and I don’t even know why I say the things that I do, I try to make sure I stand up and move around because I feel like it about to be lights out, EXTREME FATIGUE like- I need to just take a nap ASAP. most the time I am at work and I push through. my right arm has lost strength and any clothing up in my armpit and close to my neck gives me anxiety. my hands are shaky/fumbly. haven’t been able to clear my throat in a year and clear snotty/stringy mucus for months. heat intolerance. I have had MRI of brain to check for tumor/lesions and nothing. I will be making phone calls first thing tomorrow to get things figured out! surely its more than low progesterone?!?
Would I be sick all the time if this is the case? I do have some good days
Hi KT,
Breast implants serve as chronic stressors that disrupt various bodily systems, including the endocrine system which produces hormones. Adrenal fatigue and hormonal imbalances are an integral part of the illness and symptoms. The theory is twofold – toxicity and foreign body reaction (inflammation).
Both saline and silicone implants are encased in silicone shells that degrade over time. Silicone is an endocrine disruptor and has estrogenic activity and therefore this can create estrogen dominance, additionally heavy metals in breast implants are also endocrine disruptors. Altogether these factors can disrupt the adrenals, ovaries, and other hormone producing glands and commonly cause hormonal imbalances such as low progesterone, estrogen dominance, and/or diminishing of hormones along with other conditions.
Breast implants also create an inflammatory response in the body. This slows down the lymphatic system, creating stagnancy and causing the body to retain toxins, water, and fats instead of mobilizing them.
Breast implants are large foreign bodies that weigh on top of and add pressure to vital organs and this acts as physical stress, additionally the toxicity and inflammation that occurs contribute to increased stress in the body. Chronic stress results in adrenal fatigue, where the body is forced to work harder and goes into a “fight or flight” response which over time exhausts the adrenals. With adrenal fatigue the body favors cortisol production to reduce stress. Progesterone is a precursor to cortisol and is therefore reduced. Progesterone and estrogen have opposing effects and are needed to balance each other, so a deficit in one creates an imbalance. When these two are out of balance, hormone related illnesses occur. Additionally, insulin can also be affected because it works in tandem with cortisol and can lead to insulin resistance, commonly seen with PCOS.
Extreme fatigue, numbness and tingling in the arms and legs, brain fog, photosensitivity, anxiety, heat intolerance, difficulty in clearing your throat, and more are symptoms of breast implant illness.
Severity of symptoms can fluctuate, there can be good days and bad days until eventually there are more bad days than good as the body becomes exacerbated and the quality of life deteriorates. Proper explant will allow the body a chance to heal and normalize. Post explant addressing the adrenals first and supporting them should make a difference – rest, sleep, eat regularly and include protein, vegetables, and fiber with each meal, healthy fats, salt/minerals, some low carbs, and avoid spikes in blood sugar levels. For some ladies hormones will rebalance and go back to normal levels for others that are closer to menopause it may not happen without bioidentical hormone supplementation. Hormone balance is needed for homeostasis to achieve optimal health.
Five years ago my saline implants ruptured a couple months apart. I was not 65 at the time so did not have Medicare or insurance. After asking several doctors, I was told that if I wasn’t having any symptoms not to worry about it. Well, life went swiftly by and I forgot about them. Last year, I started the insurance process through Medicare only to be blocked at every turn. NOW, I’m ill. Very ill. I can barely lift my head off the pillow. Monday, I have to submit all my symptoms to the insurance company. My predicament is that the CT scan and MRI both show the implants as intact, not ruptured. So I’m being denied the visit to the plastic surgeon. Has this ever happened before. Both breasts are clearly deflated and uneven and when the implants ruptured, I peed for days. I will be paying for the initial visit out of pocket. I’m surviving on Social Security.
Is there anyone have problems from polyurethane implants?
Since they changed my old silicone to poliurathane im feeling sick.
Last month i lost my eyevision(i wear glasses to see anything), my hair(i wear false hair) and i have tons of sythomps that makes me think im going to die(it got so bad that i cant get out of bed, before these implants i was in the gym dainly 3 hours)… though my tests(2000 euro bloodtest) are fine!
Im going to explant next week, it would be nice to find sy had experience with poliurathane, i wouldnt feel so alone then…cause in usa where this information wave started prohibited the poliurathane, but europe still love to use it… 🙁
Hi Ester,
Polyurethane implants are very toxic and it is natural to develop symptoms when there is toxicity present. Research found that upon implantation the polyurethane foam disintegrates and dissolves into various chemicals including 2,4 toluenediamine (TDA), a known carcinogen. They were the first implants linked to cancer and were removed from the U.S. market in 1991 due to significant safety concerns.
“The F.D.A. data show that the dissolution of the polyurethane begins immediately after implantation and that in some cases…the coating may dissolve almost completely within five years. The greater the rate of dissolution, the greater the cancer risk, the scientists said they believed.” Source: Scientists tie breast implants to cancer (1991).
An en bloc explant would be ideal to contain the toxicity as much as possible. Please make sure the surgeon does full capsule removal, the capsules have been holding and absorbing the foam that has been disintegrating and therefore need to be entirely removed for best recovery.
Dr. Pierre Blais is a chemist and breast implant failure analysis expert with 40+ years of experience, he has written about polyurethane implants, see pages 23 “FOAM-COATED PROSTHESES – DESIGN AND RISK ISSUES” and 24 “ORIGIN OF THE POLYURETHANE FOAM IMPLANT”.
To read another story on polyurethane implants, see below under Rose Rees for her comment and the in-depth reply for more information. There are also many breast implant illness Facebook support groups you can join, here is a list.
Look at FDA.Isnt polyurethane a carcinogen??
This website has been a godsend. I have spent hours on here researching and now have some consultations set up for explant. My husband is onboard now too, which is a blessing…I have been sick for so long, it has clicked for the both of us. Thank you.
Today is the first I Learned Implant Poison was even a thing, I seen a FB post and have since been researching all day..I’m thankful for the information on this site and Thank you.. I had my saline Implants in 2006, in 2014 I had first MRI ( Due to stoke like symptom, numbing& tingling ), then 2 years later another one, I have had multiple blood test, hormone test ect. Nothing ever found. My worst symptoms are the vertigo, brain fog (Weird brain waves) muscle and bone aches, fatigue over all body weekness, loss of strength, emotional rollercoaster, blurry eyes,. constantly freezing, nausea, oh the list goes on , it is a true struggle every day… I just had surgery to remove a terrible case of endometriosis, my tubes and an ovary removed.. I also had a mammogram the results Suggested a biopsy on my left boob and the right has a small mass. AGAIN I’m thankful for the information I have been reading in this site .. I live in Utah and will look for someone to do the Metal and chemical tests and try for further help. Thank you and advise is appreciated
Hi Karen,
Welcome, the discovery of breast implant illness is a defining first step in each woman’s journey towards health recovery. Learning about proper explant with full capsule removal is the next step. Unfortunately, most tests are often found to be normal and do not reflect the level of sickness felt by women with breast implant illness. Implants create underlying inflammation and toxicity – the body’s manifestation of symptoms is the best indication of a reaction. Symptoms such as chronic fatigue, brain fog, muscle and body aches, numbness and tingling, vertigo, vision disturbances, and more are common with breast implants and tend to heal with explant. Endocrine dysfunction via adrenal fatigue and thyroid problems occur often with implants and a good measure of this is low body temperature, see here and here. Sometimes saline implants are found to have faulty valves and mold. You can read stories of other women’s experiences with this on the Saline Implants and Mold page, including a list of labs to help detect mold toxicity. You may be interested in trying the free online Visual Contrast Sensitivity (VCS) Test. MRIs are recommended to be done without contrast due to gadolinium toxicity, which can cause more symptoms. There is a Utah Breast Implant Illness FB Support Group. Popular explant surgeons in Utah are Dr. Bindrup, Dr. Hijjawi, and Dr. Crofts.
I would love to share my experience. I complained that I had a lump in my right breast. Theygive me a ultrasound. They told me I had a folder in my implant. They did not tell me that I have a leak. I have silicone implants. When I went in the next year, they said my left side was worse. Encapsuled and leaking in my lumps nodes. Went to Huntmans!! I’m a mess!! Pain everyday of my life.there is so much more. Would love to talk! Want my health back. Pain everyday and no feeling.😂
I am interested in documentation/reading material regarding Breast Implant Illness (book, reports, etc). If you know where I can obtain such information please direct me to the right source.
Thanks in advance.
Hi Regina,
The Resources page has a lot of breast implant illness information including educational links, books and other reading material. There is also a page on rheumatologist and silicone toxicity expert Dr. Arthur Brawer who writes a lot on the subject. The Expert Researchers page has material from various professionals.
I have had saline implants since 2000. Within a few months I started having numerous of the symptoms that you have listed. At that time my husband was Active Duty Military, I went to the on base doctor over and over again about a lymph node by my collar bone that was swollen, have had sporadically a rash on the bottom of my foot that doctors have given me at least 15 different diagnosis, chest pains, even panic attacks, and they literally just figured I was a hypochondriac. I would tell them, I know my body and something is just not right. At first I never even considered it was my implants. However, as the symptoms have increased over the years I started thinking this was the cause. I just heard about Breast Implant Illness yesterday and I really want an en bloc explant. I have very good insurance from two separate insurance providers but basically since this was an elective plastic surgery I am worried that I cannot get insurance to pay for the procedure. Earlier this year I had already decided that I wanted to have my implants removed and was quoted over $7,000 close to $10,000 for the procedure which I currently cannot afford. If you could provide me with any advise and assistance I would greatly appreciate it. I live in San Antonio but could easily travel to Fort Worth, Dallas or Houston, TX for the procedure.
Hi Cindy, my situation is very similar to yours. I would like to explant next year. Before meeting with a plastic surgeon, I thought it would be a good idea to schedule an appointment with a doctor of internal medicine. I will meet with her next week and am going to request some lab work and toxicity testing. Please click on this URL for BII tests that are used to investigate some fo the problems associated with breast mplants: https://www.breastimplantillness.com/tests. If any of these tests are abnormal, I will then approach my medical insurance company with the results. I am thinking that perhaps my insurance may cover the cost of explant or at least a portion of it. Just a suggestion.
Thank you so much. My health has deteriorated over the last few years and I’ve gone from incredibly fit to almost crippled. I had silicone smooth implants put in 7years ago and I just never thought they could be the cause of all my issues. I finally did some research yesterday and was gob smacked by the stories Bii that just seem to mimic my own history. I have all the same symptoms now to a debilitating degree. I have to say this page and your answers are so well informed. I’m was an intensive care nurse and you write so clearly with such a balanced voice of information and references. I do have some capsulation, no idea if I have leaks or not. I am not sure what doctors in Australia specialise in this, if you know if any I’d be very grateful. I’m going to see a very respected one in two weeks and will request explant asap. I have been feeling so hopeless at not getting answers, I don’t know if this will fix all my health problems but Just knowing that whatever happens this will make me a healthier person has really lifted my spirits.
Thank you for your wonderful, informative site. It is nothing short of a godsend. An outbreak of severe eczema on my face and neck, when I’ve never had skin issues or allergies, led me to connect the dots with a few subtler things that had happened, and an online search took me to your site. I literally found most of my answers in one place and appreciate all the effort you put into citing so many resources. It is an invaluable tool. My surgery is scheduled for next month and I’m counting the days.
I received silicone implants in late 2012. Two years later in 2014, my left implant contracted when I fell extremely ill with C Difficile Colitis and almost died. Two years later in 2016, I found out I had an active cytomegalovirus infection and extremely high cortisol. I also had low estrogen and progesterone which had caused my periods to stop for almost 3 years. To begin with, I got my breast implants to correct a congenital deformity that left me self conscious from when I first began to develop, so I guess I’ve never wanted to make the correlation to my illness. I am planning to have them redone early next year. I am doing better health wise now, however I’m still dealing with CMV, high cortisol and diet limitations due to the virus. I wonder if I were to get the implants removed if the virus were to go dormant, my cortisol would lower to normal and I could resume my old diet, again? I have done everything my integrative doctor has told me to do for CMV (anti-viral diet, supplements, etc.) and for my cortisol and while they’ve diminished, they are still very much there and affecting my life. I also suffer from right side/liver pain and do coffee enemas several times a week. If I don’t, I get very dizzy. I’ve never been able to figure out what that is about. I also suffer from other bizarre symptoms like water retention, swelling of feet and ankles and random hives. I have blamed it all on CMV up until reading your website. I feel validated. Do you think, based off what I have said, that removing my implants would benefit me? Thank you!
Hi Taylor,
Yes, removing the implants would benefit you. The implants can affect our hormones, thyroid, adrenals, gastrointestinal tract, liver, and other systems. The implants are two large foreign bodies that act as stressors to the body, they can put it in fight or flight mode and this impacts the adrenals and cortisol. There have been women in the breast implant illness groups who have had their periods stop prematurely and post explant they begin again. Your immune system is suppressed by the implants and dormant viruses reactivate and tend to stay reactivated. By removing the foreign body interference with the immune system you have a better chance to finally strengthen your immune system. The liver is another area that is impacted by the toxic overload. As the body struggles to eliminate toxins the liver becomes sluggish. Fatigue is one of the most common symptoms, post explant this generally gets better. The balance of the gut is also commonly disrupted, 70% of the immune system lies in the gut and it is referred to as the second brain. Many women, me included, have been able to resume our old diets post explant, after a period of healing. With implants I could no longer eat gluten and developed many strange allergies that all resolved post explant. There is hope, the heal is real. Removing the foreign bodies is the first step to healing.
Need help don’t no where to turn can someone please help me
Hi Joann,
Do you have any specific questions? If you need help financially, some options are:
1. Contact the Explant Financial Assistance Program.
2. If you have capsular contracture and health insurance, try to file a claim with your insurance to see if they will cover it.
3. GoFundMe or YouCaring
I had silicone implants done 5 years ago, three years ago after going to the doctor with extreme fatigue (I was sleeping 14-16 hours a day and was still exhausted) I was diagnosed with Epstein-Barr and was told there was nothing that could be done and that I would just need to let the flare up run it’s course. Deflated and exhausted I upped my Vitamin C and prayed for the best. A few months later while I was at the OB/GYN for my annual I shard with her that I was recently diagnosed with Epstein Barr but I was still so very tired, she ordered blood tests which showed I had low testosterone and Vitamin D levels. She prescribed testosterone cream and 5,000 IU’s of Vitamin D a day…to this day both of these levels remain low. Fast forward to last October, I thought I had hurt my shoulder while exercising again I went to see my doctor he prescribed a 5 day course of steroids. Not much changed after my course of meds, but now it felt like bouncing back after my workouts were taking longer and longer and I flat out just did not have the energy to complete most of them. Then in January of this year while sitting in Church, it felt as if gasoline was being poured down my arm and my hand had gone numb. Again, called and saw my doctor who sent me for an MRI which revealed a bulging disc in my neck. I have taken the prescibed meds, done the suggested treatments and still my neck and arm hurt, my hands and arms are numb and now the numbness is moving to my feet, I remain exhausted and muscle fatigue and muscle spasms are a real and painful. This morning I awoke thinking of a acquitance who recently had her implants removed due to BIL, and instantly I hoped online and found your site, I know that something is wrong with me and my doctors just keep going back to the bulging disc in my neck. I am frustrated and I am scared.
Hi Christy,
Breast implants are foreign bodies that cause a chronic foreign body reaction where your immune system is suppressed and long-term is weakened. A weakened immune system triggers dormant viruses to reactivate, such as Epstein Bar Virus (EBV), Mycoplasma, Coxsackie A, Coxsackie B, HSV-I, HSV-II, HHV 6, Cytomegalovirus (CMV), Varicella Zoster, etc. Steroids suppress the already weakened immune system, therefore they leave the body more vulnerable, and women with breast implants are generally not getting better with them. Silicone and heavy metals in the implants are endocrine disruptors and hormonal imbalances can occur. Vitamin D is a hormone and has immunemodularity activity. Vitamin D deficiency is “considered a risk factor for several chronic/inflammatory or autoimmune conditions,” see here. Not surprisingly, many women in the breast implant illness groups have low vitamin D. Your other symptoms of peripheral neuropathy (tingling in the arms, numbness in the hands) and muscle aches, pains, and spasms occur with breast implants.
The good news is that with removal of these foreign devices, women are experiencing significant health improvements. The body is able to move away from its disrupted state and begin healing. This can all be a lot to take in and absorb, but please know there is hope! Realizing something is wrong is the first step to recognizing your symptoms and a timely explant of implants and their surrounding scar tissue (capsules) is key.
Hi,
Not sure still exactly what is going on. I have been diagnosed with Scleroderma (Limited) Hashimotos, Gerd, reflux, fatigue, digestion issues, recent 30 lb weight loss.My Gp says to me as of Last Friday he thinks it’s my implants. I am 57 with 17 year old saline implants. Expalnt surgery is scheduled for Sept 28th. Not quite sure what to think. For some reason I was ok with the Ssc diagnosis but this BII business has me all freaked out. So scared they are going to find mold.
I have been so sick for so long and this all makes so much sense.ugh
Sylvia,
you are not alone. I also have Hashimoto’s. I am having explant the same day. Good luck
What great reading and useful and informative shares and replies. BII is very new to me. 35 years ago I had Surgitek implants put into my body. I was told by surgeon that I was getting new, state of the art implants, would last me a lifetime and would feel better about the new look…..TODAY I know different. I have 3 adult children , breastfed before implants were placed. Thank goodness for that. OVer the 35 years, I had a hysterectomy – I had irregular bleeding and was advised this would stop the bleeding. I have multi modular goiters, thyroid problems, hashimoto thyroiditis. Hiatal hernia removed 4 yrs ago. INvoluntery spasms in throat area, gastro problems, Uncontrolallable coughing, choking sensation, heartburn, hot and cold flashes, vagina dryness, mouth dryness, fungal issues on toes. Asthma, sensitive and allergic to many things. I have many of the symptoms of BII that are listed. All of my symptoms are and have been treated with medication from my physician. I have been recently involved with 2 motor vehicle accidents, I was t boned in Jan. 2016 and rear ended in June of 2016. BOth accidents continue to be problematic. I continue to be treated by chiro and physio. From the first accident, when I was being treated, my breast area is and continues to be an area where pain is 10 out of 10. It was then that I felt my implants were ruptured. MAmmeograms, ultra sounds showed everything was ok, or so my dr. told me. I am 65 and devastated about what I have read about BII. After 35 yrs, I must have black mouldy bags in me. Will it be safe at my age to explant? I look forward to your reply. Grateful for you and all you do. Xoxo
Hi Rose,
Very happy you are here, welcome!
Surgitek was a subsidiary of Bristol-Myers Squibb, the breast implants were sold under the names Meme and Replicon and they were polyurethane coated (very dangerous/toxic and linked to cancer). They were in production from about 1980 to when the manufacturer voluntarily withdrew them in 1991 due to significant safety concerns. They were made with aggressive chemicals on the surface to deter and weaken the immune system from forming scar tissue, thus they were a “new” attempt to reduce capsular contracture (a frequent problem with earlier implants). Among other things, adverse reaction reports indicated that the foam disintegrated and dissolved into the body, meaning it broke down into fragments and/or was “partially digested,” to the point where the foam surface disappeared – explanted implants in some cases had little to no coating left on them. Research found the polyurethane foam to breakdown into 2,4 toluenediamine (TDA), a known carcinogen. It was the first time breast implants were linked to cancer, which is interesting to note because modern textured implants are the second time breast implants have been linked to cancer, and what they both have in common is a chemically abrasive fuzzy surface.
“The F.D.A. data show that the dissolution of the polyurethane begins immediately after implantation and that in some cases…the coating may dissolve almost completely within five years. The greater the rate of dissolution, the greater the cancer risk, the scientists said they believed.” Source: Scientists tie breast implants to cancer (1991).
Silicone and polyurethane are used as sealants in cars and as toxic pesticides, yet they were introduced in breast implants as “safe, new and innovative” ideas until women were significantly harmed. You can read the marketing history of implants and using the term “new” to describe each generation of implants and then later finding out their failures, under Safety.
Mammograms and ultrasounds have had many false reports regarding suspected ruptures. It is always good to request your medical records and look through the notes, patients are often surprised by the discrepancies or new information they find. Additionally, Surgitek has some of the higher rates of ruptures amongst the earlier implants.
It is good that you explant preferably en bloc to contain as much of the rupture as possible. I would highly recommend looking into Dr. Feng or Dr. Chun as explant surgeons experienced with more complex cases. Please seek advice from your surgeon regarding your age and individual circumstances. Your body has been fighting the toxicity from these implants for a long time.
You can read a previous explant story of a 62 year old woman with ruptured Surgitek breast implants and her post explant updates, here. She states: “It’s been two weeks now and I am so very glad to be explanted” and “It has been one year since Dr. Feng [explanted] the old,old surgitek implants. What a good decision it was. I am happy to have my health and no worries about implants even if I have small breasts again.”
Hi Rose,
Your comment sent on March 31st 2018 has been received. Three attempts have been made to reach you in regard to your enquiry but twice the emails were sent back with an error message. Do you have another email address to be reached at? Or you can contact the site at [email protected].
Hello, I have had my saline, textured implants for 17 years. just over a year ago started experiencing strange symptoms that, since discovering this illness exists, seem to tell the tale. I was diagnosed with mono last year this time and since have battled additional symptoms along with this virus. Just this week, my GP drew blood to check my mono titers again and they came back elevated even more than last year! I stay so sick all the time. I can’t go for my daily runs and have very little energy to make it through my 8-5 desk job, after being a very active woman since my teens! My breasts are often very tender. My last mammogram was almost unbearable. The tech who performed it was shocked that I had so much pain during the procedure. At that time, I just knew she’d see something ominous in the x-rays. I did have to return for an ultrasound but they found nothing suspicious. Of course they weren’t looking for implant damage. My lymph nodes stay swollen, I have head aches, lower back and lower ab pain, consistent pains in my legs. I have sever drenching night sweats that are profoundly different from the hot flashes I had before I started wearing a hormone patch. I am post menopausal x 3 years and I’m 53 years old. I still have all my reproductive organs. I have a hard time with my cognizant skills to the point where, just today, I forgot my own social security number and had to locate my card to recall it. I have NEVER had problems focusing like this. I’ve no energy….I had a stress test this past week, (another way my GP is trying to pacify me), I had an EKG change but apparently it’s not life threatening: I’ve had no call back rom my doctor. He hasn’t addressed very many symptoms because I think he believes I’m being overly dramatic. I KNOW my symptoms are real. Do any of you know a proper explantation doctor in the North Alabama area? I’m not above asking my OB/GYN on my next routine visit, which is the end of August, but if you know of someone, I’d welcome the info. Thanks for such an informative article. Now I see I’m not alone…..and NOT crazy! I am almost 100% positive my illness is more from my implants than from mono. My breasts have changed shape as well. Maybe a leaky implant?
Hi Karen,
Yes, your symptoms are real and you can rest assured they align with the experiences of decades of other women. Symptoms of chronic fatigue, brain fog, swollen lymph nodes, night sweats, pains, reactivated viruses, and more, are part of a cascade of effects that occurs with breast implants.
Textured saline breast implants are problematic for a variety of reasons.
First, as large foreign bodies they stimulate a chronic foreign body reaction. Eventually this overwhelms and impairs the immune system, creating immune dysfunction and autoimmune like symptoms. Immune dysfunction allows opportunistic pathogens to grow out of control (ex. candida) and for dormant viruses to reactivate (Epstein-Barr virus, cytomegalovirus, etc). The viral titres will stay elevated because the body has a compromised immune system fighting the implants and the toxins released, and therefore becomes weakened. When it is weakened it can no longer regulate the dormant viruses. A cascade of dysregulation develops. The weakened immune system begins to overlap and affect other systems – the gut (70% of the immune system lies in the gut), the endocrine system (adrenals, thyroid, ovaries, etc), the liver, the brain, etc. – because everything in the body is interconnected.
Second, many saline implants have faulty valves that allow body fluid/tissue in and allow colonization of fungal microorganisms inside the implant. These microorganisms produce metabolites which are toxic to us known as biotoxins. There are some tests you can do to measure your body’s reaction to see if biotoxins or mold are present, such as: the free online Visual Contrast Sensitivity (VCS) test, C4a and Alpha MSH (lab work), and the brain MRI NeuroQuant test (without contrast).
Third, textured implants are the most linked to causing ALCL, a rare cancer of the immune system. The rough and textured surfaces aggravate the immune system and trigger inflammation. The textured shells can flake off and the debris can get incorporated into the capsules and lymph nodes. If you have swelling, seroma, enlarged lymph nodes, breast mass, or even capsular contracture you should request the CD30 ALCL testing for pathology. Don’t assume the doctor will test for fluid collection or that he will send capsules off for the CD30 testing, these can easily be discarded unless requested by the patient.
Mammograms are not recommended to do with breast implants because they can contribute to ruptures and they can sometimes increase symptoms. See here for a study by FDA scientists indicating that silicone or saline implants sometimes rupture or can cause pain when women undergo mammograms.
The Explant Surgeons page lists two surgeons in Alabama and seven in nearby Georgia. There have been some ladies that have been very happy with Dr. Bahair Ghazi in Atlanta.
I forgot to mention, I will be 80 in August and I am concerned because of my age.
I have had an implant since my breast cancer in 2009 and have had pain ever since. I go to my plastic surgeon every year and he says everything is okay. For the last couple of months I have been having rapid heart beat and my cardiologist said my heart is okay he said it is anxiety. Could this be my implant. Who is a good doctor to remove this in Lady Lake, FL?
Hi Mary,
Yes, ladies with breast implants have developed pains, arrhythmias, palpitations, and increased anxiety that reverse with explant.
In Dr. Brawer’s ‘Chronology of systemic disease development in 300 symptomatic recipients of silicone gel-filled breast implants,’ 77% of women had early chest pain and 25% of overall women later reported palpitations.
Test results tend to come out within the normal ranges even for the most symptomatic ladies. Therefore, symptoms tend to elude the doctors and misdiagnosis is common.
Breast implants create artificial disturbance in the body that over time creates imbalance and can result in various nutrient deficiencies. In particular, magnesium deficiency has been increasingly seen and this can cause heart arrhythmias. My recommendation for your rapid heart beat would be to speak with a functional doctor or naturopath about magnesium taurate and the antioxidant CoQ10 (in the active ubiquinol form, not ubiquinone) for the heart. You can read more about magnesium’s role in heart health here and the different forms of magnesium here. You can also research “magnesium deficiency and arrhythmia” and many results will come up.
For your age concerns I would speak to your doctor or consult with a surgeon. Please refer to the list of Explant Surgeons. Dr. Barnett in Sarasota, FL comes highly recommended by women.
Wow, I want to thank you so much! This website has been extremely informative for my daughter and me while dealing with her symptoms consistent with breast implant illness. You describe it as a “silent epidemic” and that’s why it took us so long to realize the connection with her breast implants. Now we are moving forward with explantation. We finally have hope to help my daughter’s symptoms which have been going on for over a year now and have prevented her from working. I hope many other women struggling with this same issue will be able to use this excellent resource. Thank you again for bringing awareness.
Hi Janet,
My best of wishes for your daughter to recover her health and be able to work again.
Thank you for your kind comment, it is deeply appreciated.
I also have reflux, digestive problems I am worried about my age 73 yrs to have suegery
I have had saline implants for 43 years. I hace had my galbladder out, I experience muscle pain have shortness of breath which is copd, have had to take pills to sleep for years . Does insurance pay for any of the removal?
Hi Diane,
Generally, the insurance criteria focuses on capsular contractures, ruptures, and pain. It really varies with insurance plan and state. Some women have been denied on their first try and then resubmitted again multiple times and have been accepted, while others have not been able to get coverage. There are non-profit organizations who try to help ladies through the process of getting insurance approval. See the insurance page.
Digestive problems are frequently seen in the women of the breast implant illness groups. The gut is offset by the disruption of the immune system and the toxins. Eating clean, supporting the gut flora, and reducing exposure to synthetics are important in promoting gut health. There are some articles stating that sleeping pills make acid reflux worse, see here. Maybe you can talk to a functional doctor or naturopath about natural sleep remedies that are gentler on the body, such as valerian root, melatonin, or GABA.
There have been a handful of ladies with breast implants who have had their gallbladders removed. I believe the liver gets overloaded with toxins and drug residues, causing problems with the liver detoxification pathways, and therefore some of the toxins are not neutralized and are dumped in their toxic state in the gallbladder.
Chronic obstructive pulmonary disease (COPD) has symptoms of shortness of breath, chronic cough, phlegm – these resemble symptoms some women strongly develop with breast implants.
For your age concerns, it is best to speak with your doctor about the medical necessity of explant. Forty-three years is a long time to be holding stagnant water in an implant in the body. Some saline implants have faulty valves and devleop mold, they can become like dirty aquariums. But it truly depends, some come out clean.
I had my implants done approx 20 years ago. I did not develop any illnesses until approx 3 years ago. It started out slow but has progressed this past 6 months. I initially was diagnosed with RA – had three ANA test positive within 3 months. Then 2 years ago I had stomach issues develop. Gall bladder was check out. They did nuclear test and several CT scans. Everything looked normal. I have been complaining with lower abdominal pain along with low back pain. X-rays are normal. Just had a contract CT last week. Everything is normal once again. I feel like some one has hit me in my lower back with a bat. And if I had female parts it feels like major cramps.
I am thinking maybe I have major scar tissue growing inside is my lower abdomen. I don’t know Funny – as I am speaking I just got a call from the gastroenterologist to schedule an upper GI……not sure I will.
Looking for surgeon in Northwest Arkansas or in Destin FL are to consult with.
Thanks,
Pam, Destin
Hi Pam,
Many women with breast implants tend to have tests with relatively normal results despite having numerous symptoms and health concerns. They are sent through the medical circle of additional doctors/specialists and tests, with little being uncovered and yet more medications being dispensed. Some ladies are even sent through additional surgeries of having body parts removed – such as ovaries, thyroid, or gallbladder – before they realize their breast implants are foreign objects contributing to a chronic foreign body reaction and to systemic dysregulation.
Rheumatoid arthritis and high ANA’s have been discussed a lot with breast implants. Some women have experienced reversal of RA and other autoimmune conditions with explant. You also mentioned stomache issues – gut health is paramount to restoring the equilibrium that is often offset by the implants. How the gut is harmed and how to support it are discussed on the healing page. The gut is intimately connected with the immune system, hormones, detoxification, and more. The intestinal walls absorb toxins and contribute to inflammation. When the immune system is weakened the gut is also affected. Gastrointestinal and digestive problems such as leaky gut, gut dysbiois, irritable bowel syndrome, bowel inflammation (colitis), allergies/food intolerances, parasites, fungus (candida) and more, are increasingly being seen in women in the breast implant illness groups. Tests with contrast are not recommended as these use gadolinium, which can cause gadolinium toxicity (one of the many symptoms is aching pain, which it sounds like you are experiencing after the contrast CT) and can contribute to further detoxification issues (which many are already experiencing), therefore it adds to the toxic overload.
The Explant Surgeons page lists two plastic surgeons in Arkansas and many in Florida. The closest one near Destin is Dr. Tara Harden in Fort Walton Beach. There have been a handful of ladies that have said she does the correct process of doing a proper explant, sending capsules to pathology, submitting to insurance, and have been happy with their results. In Florida there is also Dr. Marguerite Barnett in Sarasota who has many good word of mouth recommendations from ladies.
There are breast implant illness Facebook support groups for Arkansas and Florida where you can engage with other ladies in those regions who are exploring explant surgeons and going through explantation.
I was told there are two test to be done to determine if the implants need to be removed. What are they?
Thank you for your help.
Pam
There is no definitive test(s) for breast implant illness. There used to be silicone antibody tests and a variety of other immunological tests, but they for the most part have been discontinued.
If you are interested in seeing if you have a sensitivity to silicone, currently available is a popular test called the Silicone Hypersensitivity Panel and it measures sensitivity to: Silicone, Silicates (silicon dioxide), Polyvinylpyrrolidone, Tin/stannous chloride, Titanium dioxide, Petroleum by products, Xylene, Toluene, Benzene, Latex, Phenol, Formaldehyde, Vinyl chloride, Green #5, Blue #2, Violet #2, Aspergillus niger, Candida albicans, Aluminum. You can read more information about silicone allergy testing here.
Ultimately, if you are having symptoms it is really important to listen to your body and to respond in a timely manner to prevent further progression of illness. Explant is the first step towards health by removing artificial disturbance and interference with the body.
Has anyone experienced feeling hot and cold. It almost feels like an infection but there is no fever and all blood work is normal. I have exhausted and and all doctors and no one seems able to explain it. This odd symptom began around a year after a double mastectomy and silicone breast implant reconstruction. I also have other symptoms similar to what others have talked about on this page. Mainly memory problems and breathing difficulties. Last year I was diagnosed with Cutaneous T-cell lymphoma. I had no idea there were so many problems with implants until I came across this page. Thank you so much:
Hi EllieMCM,
You may have a biofilm related infection. Biofilm is adherent bacteria that commonly forms a film covering the surfaces of biomaterials in the body. They cause chronic low grade bacterial infections, chronic inflammation, and can cause capsular contracture. They are almost undetectable and highly resistant to antibiotics – antibiotic treatment alone without implant removal can actually increase resistance of the biofilm. This underlying infection is treated with removal of the implant. There is a research article from 2015, titled “Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma” that found “chronic biofilm infection around breast prostheses produces an increased T-cell response both in the pig and in humans.” You may want to ask your doctor if this is playing a role in your Cutaneous T-cell lymphoma.
Breast implants have been confirmed to cause cancer. In March 2017, the FDA issued a warning confirming that breast implants cause Anaplastic Large-Cell Lymphoma (ALCL), a rare type of blood cancer. Dr. Mark Clemens, a plastic surgeon who has been following ALCL, stated “The current lifetime risk of BIA-ALCL in the U.S. is estimated to be 1:30,000 women with textured implants based upon current confirmed cases and textured implant sales data over the past two decades.” This statement can be found here. As a note, both smooth and textured breast implants can cause ALCL.
This is so weird to me because I had mine done in 2007 and in 2009 I started having all kinds of symptoms . Was told lunes disease then ms after testing negative was sent to Mayo Clinic had a positive Ana and abnormal blood work . Now I have lupus and chrons can’t work and have no energy . Would love to be able to have them out but do not have the money ..
Hi Nicole,
Positive ANA and debilitating fatigue/no energy are commonly seen with breast implants. Lupus has been evidenced too.
Breast implant illness can mimic many illnesses and cause misdiagnoses. Some ladies were misdiagnosed with MS, lupus, or rheumatoid arthritis when they actually had breast implant illness and then after explant are recovering or are recovered. Other times the severity of the damage that breast implants cause is longer lasting and women do develop lupus and other autoimmune conditions. Essentially, the body mounts a persistent immune attack (first acute and then chronic) on the foreign objects until often times it eventually starts to attack itself. The implants are a constant source of immune stimulation, this involves the release of many inflammatory mediators that result in inflammation, which has the potential to create symptoms in every organ system. You can read more about this under mechanisms. The first step towards improving your health is to remove all foreign interference within the body, beginning with explantation and then followed by diet, detoxification, and treating any other areas that have been affected (such as co-infections, mold/fungal issues which are common with breast implants, thyroid/adrenals, etc). After explant you can also remove any other foreign material such as potential mercury amalgams. In regards to financial support, you can try to see if insurance can be of assistance or do a go-fund me.
I’m completely freaking out! I have been to so many doctors for joint pain, bones aching, tired when I shouldn’t be, brain fog, I have had mammograms, sonograms, rheumatoid dr, cortisone shots for pain. I have saline implants & have had them for at least 20 years. I have had no complications with them so was advised to just leave them alone instead of replacing them. I have known about silicone leakage but wasn’t concerned because mine are saline now I’m worried!
I have a rupture, for the third time in my saline breast implants. I have a consult next week to decide if I want to remove or replace them. Do saline implants cause “breast implant illness”? I know the shell is silicone but what is leaking into my body is slat water. Any suggestions?
Hi Jennifer, good question – yes, saline and silicone breast implants both cause breast implant illness. Saline implants are large foreign objects in the body that contribute to an inflammatory, chronic foreign body response and will also have the potential risk of biotoxicity.
With saline implants there is the added element that many of the saline valves are permeable and allow body fluid/tissue in and allow colonization of microorganisms inside the implant. These microorganisms produce metabolites which are toxic to us known as biotoxins (including mold), which produce diverse inflammatory and toxic responses.
Many saline implants have defective faulty valves that leak and cause a “backwash” effect in the body. This allows the cultivation of mold in the saline shell where it can thrive for years, causing biotoxicity symptoms. The labels on the saline solution (pyrogen-free Sodium Chloride U.S.P. Solution for Injection) to fill the saline implants, recommends storage at 25°C (77°F) and includes a discard date of about 18 months. With these implants the saline is stored in the body at 98.6°F for many years. This makes the perfect breeding conditions for fungi and other microbes to grow. Mold from faulty valves has been recorded to be found in saline breast implants since as early as 1978. It continues to occur and is regularly suppressed and buried by the medical community. (Sources: here see pg.15 and here)
We recommend proper explant with full capsule removal, as this correlates with health recovery. Do not let them try to convince you to allow them to drain the implants in the office and then remove them at a later date. Piercing a hole in implants and then leaving them in the body in that condition leaves you vulnerable to further biotoxins and contamination. An experienced and knowledgable surgeon will be committed in full capsule removal.
Hello, I had reconstruction after a 2009 double mastectomy and have been sick ever since. I’ve been shuffled from Dr. to Dr., developed a benign tumor over 5 centimeters, suffered infection after infection, and developed gastric problems, lymph node problems, pain, swelling, ect. ect. recently I was told by an ear, nose and throat Dr. they were almost positive I suffered from implant illness. My symptoms have worsened to shortness of breath and muscle weakness, I used to run, now I can barely get off the couch. I have an appointment scheduled in May with the immunologist, but called My plastic surgeon whom I’m seeing on Monday. Your forum has help prepare me for the visit, and I Thank You. I’m scared to go through another surgery but am hopeful I can restore some of My Health. I will be My own advocate this time around. I had a massive infection and drains for over 8 weeks following My original Mastectomy. Thank You Kelly C. Massachusetts
Hi Kelly,
You have the classic symptoms of breast implant illness.
I emailed you an article on silicone immunology for any immunology concerns.
Most of us experience explant surgery to be relatively easier than implantation. The body is given a relief from the weight, toxicity, and foreign body interference of the implants, and the outcome is lighter than when we get implanted. There is a FB group called Breast Implant Illness and Breast Cancers Survivors Home that may also be of help. Holding you in love and light, please don’t hesitate to ask any questions.
Looking for a surgeon in Walnut Creek California (San Francisco closest big city)
Thanks 🙂
Hi Denette,
The Explant Surgeons page lists the following surgeon in North California: Dr. Tancredi D’Amore in Corte Madera. Also, there is a Breast Implant Illness Support California FB Group where you can see other ladies comments on surgeons and ask questions on them.
Sorry, meant explant
Can you recommend an explanation surgeon in the Wilmington, nc or charleston, sc area?
Hi Victoria,
The Explant Surgeons page lists Dr. Tracy S. Harvey in Charleston, South Carolina and four surgeons in North Carolina.
Hello, thank you so much for this website and all who shared their experiences. Can you recommend a great surgeon in Mexico who specializes in Explant in cancer patients.
Thank you!
Joe-Hannah
I have became extremely ill as soon as a new pair of implants came in my body. I called the surgeon as soon as I finished my pain killers and told him I was hurting so they said to go see a doctor something else was going on. It has been a year and I am worse than ever. Last night I decided that I need to remove my implants. I just couldn’t see my self with out breast. My illness has me feeling so sick I need them removed asap. I don’t know where to start by making sure I get the right doctor to remove them. The nurse in plastic surgeons office said they could just pull them out and under regular anistedic. I don’t see that right, I hear about making sure all is taken out and cleaned. Please if anyone know of a good doctor in Houston, please help me.
Hi Lupe,
Thank you for sharing your experience. Do not let a nurse or doctor try to tell you they can pull the implant out in the office under local anesthesia, for a quick procedure – if they do, run!
The Explant Surgeons page lists eight plastic surgeons in Houston who have done proper explant and have been recommended by ladies in the breast implant illness groups, they are:
Dr. Mark Clemens, Dr. Fabian Worthing, Dr. Paul Fortes, Dr. Shayan Isaddost, Dr. Scott Yarish, Dr. James F. Boynton, Dr. Melissa Crosby, and Dr. Kristi Sumpter. Look through the list of questions to ask them, which details all of the concerns you will want addressed by the surgeon. I would interview a few and choose the one you feel most comfortable with.
Recovery tends to be faster with people who have had the implants in for a less amount of time and with full capsule removal. In my humble opinion, you will most likely fully recover your health. Please keep in touch.
Hi does anyone know of any good surgeons that do En Bloc or Total Capsulectomy procedure in northern ireland or ireland.
Blessing xo
Hi Katie-lou, someone recommended Dr. Margaret O’Donnell in Dublin.
Her comment is: “She doesn’t use the term en bloc so I asked her to describe how she would remove and she demonstrated with a tissue and an implant and said that both would be removed together as a unit to avoid silicone spillage as i had a rupture. I asked her to remove both implants en bloc and she agreed even though she said that it probably wasn’t necessary in the un ruptured side. She took 5 hours to make sure that she got it all out and I am happy with my result. If you are looking for another surgeon I would certainly recommend her.” See here and here.
Further, I have emailed this surgeon and she replied “Thank you for your enquiry. I do both En Bloc, and Total Capsulectomy explant procedures, depending on the indication.”
If you consult with this surgeon, describe how you want the capsule and implant removed as one unit (en bloc) and alternatively ask/insist on full capsule removal (total capsulectomy). Here is a list of explant questions to ask a surgeon.
This website has been SUCH an amazing resource! Thank you for everything you do. You should really link up with Christina Roulund-Dennis, I discovered her blog post on breast implants and how they dont have to be leaking to get sick (http://christinasfitness.com/your-implants-dont-have-to-be-leaking-for-you-to-get-sick/). She answered all my questions on Facebook chat for free and has helped me immensely. You two should team up!!